| Clin Mol Hepatol > Volume 19(1); 2013 > Article |
ABSTRACT
Background/Aims
The incidence of multidrug-resistant (MDR) chronic hepatitis B (CHB) during sequential lamivudine (LAM) and adefovir dipivoxil (ADV) treatment is increasing. We investigated the antiviral efficacies of various rescue regimens in patients who failed sequential LAM-ADV treatment.
Methods
Forty-eight patients (83.3% of whom were HBeAg-positive) who failed sequential LAM-ADV treatment were treated with one of the following regimens: entecavir (ETV) (1 mg) monotherapy (n=16), LAM+ADV combination therapy (n=20), or ETV (1 mg)+ADV combination therapy (n=12). All patients had confirmed genotypic resistance to both LAM and ADV and were evaluated every 12 weeks.
Results
The baseline characteristics and treatment duration did not differ significantly among the study groups. During the treatment period (median duration: 100 weeks), the decline of serum HBV DNA from baseline tended to be greatest in the ETV+ADV group at all-time points (week 48: -2.55 log10 IU/mL, week 96: -4.27 log10 IU/mL), but the difference was not statistically significant. The ETV+ADV group also tended to have higher virologic response rates at 96 weeks compared to the ETV monotherapy or LAM+ADV groups (40.0% vs. 20.0% or 20.0%, P=0.656), and less virologic breakthrough was observed compared to the ETV monotherapy or LAM+ADV groups (8.3% vs. 37.5% or 30.0%; P=0.219), but again, the differences were not statistically significant. HBeAg loss occurred in one patient in the ETV+ADV group, in two in the ETV monotherapy group, and in none of the LAM+ADV group. The safety profiles were similar in each arm.
Chronic hepatitis B (CHB) affects 400 million individuals worldwide and is a leading cause of liver-related morbidity and mortality.1,2 Studies have demonstrated that the risk of liver disease progression in patients with CHB is associated with elevated hepatitis B virus (HBV) DNA levels.3 The introduction of oral nucleos(t)ides analogue (NA) therapy in the last two decades has revolutionized the treatment of CHB. By achieving sustained virologic suppression, long-term NA therapy has been shown to reduce cirrhotic complications and the hepatocellular carcinoma (HCC).
Unfortunately, antiviral resistance remains an important issue in long-term treatment antiviral treatment. For lamivudine (LAM), the signature rtM204V/I and rtL180M mutations occur in more than 70% of CHB patients after five years of therapy.4,5 Under these circumstances, either switching to adefovir dipivoxil (ADV) monotherapy or adding ADV to LAM has proven to be effective in treating LAM-resistant CHB and there are no significant differences between the two regimens in the suppression of viral replication during the first year of treatment.6 However, adding sequential LAM to ADV monotherapy leaves one vulnerable to developing ADV resistance and can result in the emergence of socalled multidrug-resistant (MDR) HBV strains with resistant mutations co-locating on the same viral genome.7
While the incidence of MDR HBV due to sequential LAM-ADV antiviral therapy is increasing worldwide, tenofovir-based rescue therapy has demonstrated favorable virologic outcomes.8,9 In areas where tenofovir is not yet available (e.g. Korea), such patients receive either entecavir (ETV) monotherapy, a combination of ETV and ADV, or a combination of LAM and ADV. However, relatively little is known about the efficacy of these rescue therapies in treating CHB that is resistant to both LAM and ADV.
In this study we compared the therapeutic efficacy of combination therapy with ETV and ADV to ETV monotherapy and combination therapy with LAM and ADV in CHB patients who demonstrate both LAM and ADV resistance.
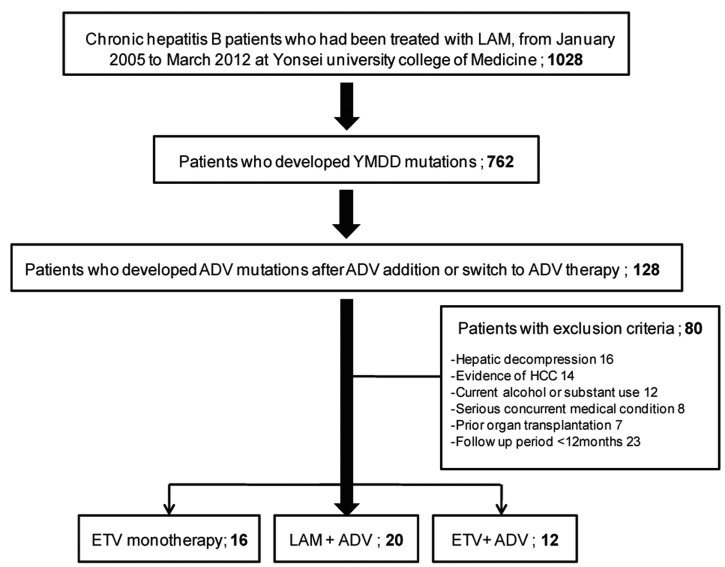
From January 2005 to March 2012, 762 CHB patients were treated with ADV switch therapy or combination therapy with ADV and LAM due to the emergence of YMDD mutations during LAM treatment at Severance Hospital, Yonsei University College of Medicine in Seoul, Korea. Among them, 128 patients who also had an ADV mutation met the criteria for this study. All patients had been positive for hepatitis B surface antigen (HBsAg) for at least six months prior to antiviral treatment.10
The exclusion criteria were as follows: a history of hepatic decompression including presence of ascites, variceal bleeding, or hepatic encephalopathy at enrollment (n=16); a history of HCC at enrollment (n=14); concomitant liver disease other than CHB including autoimmune hepatitis, coinfection with hepatitis C and D virus or human immunodeficiency virus (HIV) (n=8); alcohol ingestion in excess of 40 g/day for more than five years (n=12); a history of liver transplantation (n=7); and follow-up period less than 12 months (n=23).
The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System. Prior to enrolling in this study, patients provided informed consent by agreeing to participate and signing an Informed Consent Form.
All patients were seen at baseline and at 3, 6, 12, 18, and 24 months, and routine blood chemistry tests, serum HBV DNA level, and serologic markers (HBeAg/anti-HBe in the case of HBeAg-positive CHB) were assessed at all time points. HBsAg, HBeAg, and antibody to HBeAg (anti-HBe) were tested by enzyme-linked immunosorbent assay (ELISA; Abbott Laboratories, Chicago, IL, USA) and serum HBV DNA levels were quantified using a commercially available real-time polymerase chain reaction (PCR) assay (COBAS AmpliPrep-COBAS TaqMan HBV test, Roche) with a linear detection range of 20-170,000,000 IU/mL. The serum alanine aminotransferase (ALT) level was measured using standard laboratory procedures with the upper limit of normal set at 46 IU/mL.
Primary non-response to antiviral therapy was defined as a <1 log10 IU/mL reduction in HBV DNA concentration at three months. Virologic response was defined as an undetectable HBV DNA level (<20 IU/mL).11 Virologic breakthrough was defined as a confirmed increase in HBV DNA level of >1 log10 IU/mL from the nadir during antiviral therapy.12 A biochemical breakthrough was defined as an increase in ALT to greater than the upper limit of normal in accordance with virologic breakthrough after initial normalization.13 Antiviral resistance to LAM or ADV was defined genotypically using restriction fragment mass polymorphism (RFMP), as previously described.14,15
The primary objective of this study was to evaluate the virologic response to rescue regimens. The secondary goal was to evaluate the serologic and biochemical response, virologic breakthrough, and safety profiles of each regimen.
Continuous variables were compared using a two-tailed Student's t-test (and if appropriate, Mann-Whitney test) or a one-way ANOVA method (and if appropriate, Kruskal-Wallis method). The repeated-measures ANOVA method was used to evaluate the differences between mean HBV levels for each regimen. Categorical data were compared using the Chi-squared test or Fisher's exact test.
Statistical analyses were performed using SPSS software (ver. 18.0; SAS Institute Inc., Cary, NC, USA). A value of P<0.05 was considered statistically significant.
A total of 48 patients were included in this study. Sixteen patients were treated with ETV monotherapy (1 mg/day; ETV monotherapy group), 20 patients were treated with a combination of LAM (100 mg/day) and ADV (10 mg/day) (LAM+ADV group), and 12 patients were treated with a combination of ETV (1 mg/day) and ADV (10 mg/day) (ETV+ADV group) (Fig. 1). The characteristics of each group are shown in Table 1. There were no significant differences in characteristics between the three treatment groups, including age, gender, serum ALT, and HBeAg positivity rate. The median duration of antiviral treatment was also similar among the groups: 23.93 months in the ETV monotherapy group, 19.30 months in the LAM+ADV group, and 18.37 months in the ETV+ADV group. Baseline serum HBV DNA levels were 6.08 (range 2.09-8.04) log10 IU/mL, 4.92 (range 2.34-7.77) log10 IU/mL, and 5.52 (range 2.54-8.23) log10 IU/mL, respectively (P=0.223).
The virologic outcomes including virologic response, biochemical response, and serologic response in the three treatment groups are illustrated in Table 2. Patients in the ETV+ADV group tended to demonstrate a higher virologic response at 24 months (40.0%) although the data failed to show statistical significance (P=0.656). Among patients who had high baseline ALT levels, the proportion of patients with ALT normalization did not significantly differ among the three groups (100.0%, 90.0%, and 100.0%, respectively). Among patients who were HBeAg-positive at baseline, 16.7% of the ETV monotherapy group, 0% of the LAM+ADV group, and 9.1% of the ETV+ADV group achieved HBeAg loss.
The mean reduction and mean changes in serum HBV DNA levels over 24 months in the three groups are shown in Table 3 and Figure 2. The decline of serum HBV DNA from baseline tended to be the greatest in the ETV+ADV group at all time points (12th month: -2.55, 24th month: -4.27 log10 IU/mL) although no statistical significance was found (all P>0.05).
All primary non-response and virologic breakthroughs are illustrated in Table 4. In total, 13 patients experienced virologic breakthroughs during the 24 months of treatment. Among them, six (37.5%) belonged to the ETV monotherapy group and six (30.0%) belonged to the LAM+ADV group, while only one patient (8.3%) was part of the ETV+ADV group. The rate of primary non-response tended to be higher in the LAM+ADV and ETV+ADV groups than in the ETV monotherapy group, but the difference was not statistically significant (P=0.219). Among 6 patients with virologic breakthroughs who were previously managed with ETV monotherapy, 4 patients (66.7%) showed genotypic ETV resistance (two in rtS202 and two in rtT184) in conjunction with biochemical breakthrough.
ADV has shown to be efficacious and safe for up to five years in HBeAg-positive and HBeAg-negative CHB patients with LAM-resistance. However, several studies have reported that ADV resistance mutations frequently develop in LAM-resistant patients after switching to ADV monotherapy.14,16,17 Therefore, current international guidelines recommend initiating combination therapy rather than switching to ADV monotherapy. In many countries within HBV endemic areas, including Korea, many patients have already received long-term LAM and ADV sequential monotherapy due to the relatively low cost and limited subsidization from the Korean health insurance system. Thus, investigations are needed to find an effective rescue therapy for patients who have experienced treatment failure with sequential LAM-ADV.
The best option for such patients is tenofovir-based combination therapy. Tenofovir, a new nucleotide analogue licensed in 2008 for the treatment of HBV infection in Europe and the United States, is efficacious against wild-type and LAM-resistant HBV, both in vitro and in vivo.18 A recent study showed that tenofovir had significant activity against HBV in patients with a high rate of genotypic resistance (rtM204I/V, rtA181T/V, and rtN236T mutations), including in those patients who failed sequential LAM and ADV monotherapy.19 The American Association for the Study of the Liver Diseases (AASLD) practice guidelines from 2009 recommend that a combination therapy of tenofovir and ETV be used in patients with sequential LAM and ADV treatment failure.11 Likewise, the Asian Pacific Association for the Study of the Liver (APASL) guidelines from 2012 recommends a combination of ETV and tenofovir.20 Unfortunately, tenofovir is unavailable in many Asian countries. Therefore, we conducted this study by comparing the currently available antiviral agents in Asia to test the assumption that combination ETV and ADV therapy may be a better, or at least comparable, option compared to ETV monotherapy or combination LAM and ADV therapy.
In the present study, ETV+ADV combination therapy tended to more effectively suppress viral replication compared to either LAM+ADV combination therapy or ETV monotherapy in patients with both LAM and ADV resistance. Although there were no statistically significant differences, the ETV+ADV group showed a higher virologic response at 24 months, a greater reduction of mean serum HBV DNA levels at 12 and 24 months, and lower virologic and biochemical breakthroughs. Other recent studies have also shown that the addition of ADV is superior to switching to ETV monotherapy in LAM-resistant CHB.21,22 The long-term efficacy of ETV monotherapy may be limited primarily due to frequent emergence of ETV resistance in ADV-refractory CHB patients with prior LAM resistance, while emergence of ETV resistance in treatment-naïve patients is very rare.23 Accordingly, in our study, six patients (37.5%) in the ETV monotherapy group demonstrated virologic breakthrough and among them, four patients had confirmed ETV resistance (two in rtS202 and two in rtT184). Furthermore, since CHB patients with sequential LAM and ADV resistance often have resistant mutations to both drugs on the same viral genome,24 the combination of LAM and ADV demonstrated expectedly unsatisfactory antiviral efficacy. Indeed, recent studies have shown that combination therapy with LAM and ADV is ineffective and inferior even to ETV monotherapy.25 In this study, the ETV monotherapy group showed better results regarding the reduction of HBV DNA during the first six months of therapy compared to the LAM+ADV group. However, after six months of treatment, the ETV group was most prone to developing virologic and biochemical breakthrough. In the early period, ETV monotherapy had better efficacy than both the LAM+ADV or ETV+ADV groups due to suppression of ADV resistance, but the effect decreased as time passed with previous LAM resistance.
This study has some limitations. Firstly, since there was a relatively small sample size, the superiority of ETV and ADV combination therapy was not clearly demonstrated despite the tendency toward better virologic outcomes (perhaps due to type II statistical error). Thus, physicians should exercise caution in interpreting these results, and further validations are required to resolve this issue. Secondly, since tenofovir is currently not available in Korea, we were unable to evaluate the efficacy of the tenofovir-based regimen. Further research is needed to evaluate more efficient combination therapies that include tenofovir. The combination therapy of ETV and ADV led to the virologic responses in 40% of subjects at most, thus further studies are needed with more potent antiviral agents such as tenofovir.
In conclusion, for patients in Korea with both LAM and ADV MDR mutations, combination therapy with ETV and ADV tended to have a better virologic response compared to ETV monotherapy or combination therapy with LAM and ADV.
Acknowledgements
This work was supported by the grant from the Bilateral International Collaborative R&D Program from the Ministry of Knowledge Economy and by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Abbreviations
AASLD
American Association for the Study of the Liver
ADV
adefovir dipivoxil
ALT
alanine aminotransferase
anti-HBe
antibody to HBeAg
APASL
Asian Pacific Association for the Study of the Liver
CHB
chronic hepatitis B
ELISA
enzyme linked immunosorbent assay
ETV
entecavir
HCC
hepatocellular carcinoma
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
HIV
human immunodeficiency virus
LAM
lamivudine
MDR
multidrug-resistant
NA
nucleos(t)ides analogue
PCR
polymerase chain reaction
RFMP
restriction fragment mass polymorphism
REFERENCES
1. Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004;11:97-107. 14996343.


2. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 2006;43(2 Suppl 1):S173-S181. 16447285.


3. Ahn SH, Chan HL, Chen PJ, Cheng J, Goenka MK, Hou J, et al. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int 2010;4:386-395. 20305758.



5. Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003;125:1714-1722. 14724824.


6. Chen Y, Ju T. Comparative meta-analysis of adefovir dipivoxil monotherapy and combination therapy of adefovir dipivoxil and lamivudine for lamivudine-resistant chronic hepatitis B. Int J Infect Dis 2012;16:e152-e158. 22226087.


7. Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology 2006;44:703-712. 16941700.


8. Kuo A, Dienstag JL, Chung RT. Tenofovir disoproxil fumarate for the treatment of lamivudine-resistant hepatitis B. Clin Gastroenterol Hepatol 2004;2:266-272. 15017612.


9. van B├Čmmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 2010;51:73-80. 19998272.


10. Chon YE, Kim SU, Lee CK, Heo J, Kim JK, Yoon KT, et al. Partial virological response to entecavir in treatment-naive patients with chronic hepatitis B. Antivir Ther 2011;16:469-477. 21685534.


12. European Association For The Study Of The Liver. EASL clinical practice guidelines. Management of chronic hepatitis B. Gastroenterol Clin Biol 2009;33:539-554. 19481389.


13. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007;45:1056-1075. 17393513.


14. Lee JM, Park JY, Kim do Y, Nguyen T, Hong SP, Kim SO, et al. Long-term adefovir dipivoxil monotherapy for up to 5 years in lamivudine-resistant chronic hepatitis B. Antivir Ther 2010;15:235-241. 20386079.


15. Han KH, Hong SP, Choi SH, Shin SK, Cho SW, Ahn SH, et al. Comparison of multiplex restriction fragment mass polymorphism and sequencing analyses for detecting entecavir resistance in chronic hepatitis B. Antivir Ther 2011;16:77-87. 21311111.


16. Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology 2004;126:81-90. 14699490.


17. Ryu HJ, Lee JM, Ahn SH, Kim do Y, Lee MH, Han KH, et al. Efficacy of adefovir add-on lamivudine rescue therapy compared with switching to entecavir monotherapy in patients with lamivudine-resistant chronic hepatitis B. J Med Virol 2010;82:1835-1842. 20872709.


18. Benhamou Y, Tubiana R, Thibault V. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N Engl J Med 2003;348:177-178. 12519935.


19. Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut 2011;60:247-254. 21036792.


20. Ahn SH, Chan HL, Chen PJ, Cheng J, Goenka MK, Hou J, et al. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int 2010;4:386-395. 20305758.



21. Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007;45:307-313. 17256746.


22. Inoue J, Ueno Y, Wakui Y, Niitsuma H, Fukushima K, Yamagiwa Y, et al. Four-year study of lamivudine and adefovir combination therapy in lamivudine-resistant hepatitis B patients: influence of hepatitis B virus genotype and resistance mutation pattern. J Viral Hepat 2011;18:206-215. 20367795.


23. Kwak MS, Choi JW, Lee JS, Kim KA, Suh JH, Cho YS, et al. Long-term efficacy of entecavir therapy in chronic hepatitis B patients with antiviral resistance to lamivudine and adefovir. J Viral Hepat 2011;18:e432-e438. 21914060.


Figure┬Ā2
Changes in serum HBV DNA levels from baseline during the treatment period in the ETV monotherapy group, and the LAM+ADV and ETV+ADV combination therapy groups.

Table┬Ā1.
Patient characteristics at baseline
Table┬Ā2.
Comparison of virologic responses, biochemical responses, and serologic responses in the three treatment groups*
Table┬Ā3.
Comparison of mean serum HBV DNA levels and mean reductions therein in the three treatment groups*
Table┬Ā4.
Comparison of rates of primary nonresponse, virologic breakthrough, and biochemical breakthrough in the three treatment groups*



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




