| Clin Mol Hepatol > Volume 29(2); 2023 > Article |
|
See the commentary-article "Management of hepatocellular carcinoma in China: Seeking common grounds while reserving differences" on page 342.
See the commentary-article "The clinical management of hepatocellular carcinoma in China: Progress and challenges" on page 339.
See the commentary-article "The clinical management of hepatocellular carcinoma in China: Progress and challenges" on page 339.
ABSTRACT
Liver cancer is the fourth most prevalent and the second most lethal cancer in China. Hepatitis B virus (HBV) infection represents a major risk factor for hepatocellular carcinoma (HCC). Liver ultrasonography plus alpha-fetoprotein every 6 months continues to be the predominant surveillance modality. The age-Male-ALBI-Platelets score was recommended in the recent 2022 Chinese guidelines to predict HCC occurrence. The Chinese liver cancer (CNLC) staging system proposed in the 2017 guidelines continues to be the standard model for staging with modifications in the treatment allocations. Considering the aggressive nature of HBV-associated HCC, multimodal and high-intensity strategies like the addition of immunotherapy-based systemic treatment to local therapies, including resection, ablation, and intra-arterial therapies, have been adopted in real-life practices in China. The latest Chinese guidelines recommend atezolizumab plus bevacizumab, suntilimab plus a bevacizumab analog, lenvatinib, sorafenib, donafenib, and FOLFOX (folinic acid, fluorouracil, and oxaliplatin) chemotherapy as first-line treatment without priority. Regorafenib, apatinib, camrelizumab, and tislelizumab have been added as second-line systemic therapies for patients who progressed on sorafenib. Systemic therapies adopted in real-life practice are sophisticated with various combination modalities and different sequences.
Liver cancer is the fourth most prevalent and the second most lethal cancer in China [1,2]. Approximately 410,000 patients were newly diagnosed with liver cancer in China in 2020 (https://gco.iarc.fr), accounting for 45.3% of new global cases [3]. Hepatocellular carcinoma (HCC) constitutes the majority of primary liver cancer, accounting for 75ŌĆō85% of total cases. Hepatitis B virus (HBV) infection represents the major risk factor for HCC in China. According to a previous report, 69.9% of Chinese patients with HCC had a background of HBV infection, 5.2% had hepatitis C virus (HCV) infection, and 5.8% had both [4]. Other risk factors include aflatoxin exposure, alcohol abuse, and metabolic disorders. Since 1992, the neonatal HBV vaccination program [5] and effective anti-viral agents have contributed to a significant decline in HCC incidence, especially for those below 40 years old [6]. HBV-associated HCC belongs to a molecular subtype named proliferation subtype and is featured by poor differentiation and high aggressiveness [7]. According to the BRIDGE (Bridge to Better Outcomes in HCC) study, only 36% of Chinese cases were initially diagnosed at the early stage and eligible for curative treatments, while the remnant 9% and 55% were at an intermediate and advanced stage, respectively [8]. Efforts to improve the diagnostic sensitivity and the therapeutic efficacy of HCC treatment have contributed to a decrease of 20.3% in age-standardized mortality in China from 1990 to 2017 [1].
Patients with chronic HBV/HCV infection, cirrhosis of any causes, alcohol abuse, non-alcoholic steatohepatitis, or family history of HCC are listed as a high-risk population for developing HCC, especially among men over 40 years. The age-Male-ALBI-Platelets (aMAP) score has been added to the 2022 Chinese guidelines to help discriminate a high-risk population for HCC occurrence (aMAP score >60 with an annual HCC incidence reaching 1.6ŌĆō4%) [11]. For these high-risk populations, ultrasonography (US) plus alpha-fetoprotein (AFP) should be carried out every 6 months.
Considering the social cost-effectiveness in a country with a large HBV-infected population, liver US plus AFP every 6 months continues to be the predominant surveillance modality in China. In Japan where over 70% of HCC patients are initially diagnosed at an early stage [8], liver US plus AFP, desgamma-carboxy prothrombin, and AFP-L3 testing are performed every 6 months for high-risk groups (HBV/HCV infection, cirrhosis of other etiologies) and every 3ŌĆō4 months for extremely high-risk groups (HBV/HCV related cirrhosis) [12]. Apart from insufficient testing items and surveillance frequency, the lack of government-supported programs to cover surveillance for more populations also contributes to a relatively low diagnostic rate of early HCC in China. The integration of resources from the community into the hospital to establish an efficient surveillance and call-back system is expected to resolve such a dilemma. In addition, new blood-based biomarkers including a 7-miRNA panel [13] and GALAD (gender, age, AFP-L3, AFP, DCP) score14 have been developed to assist in the early detection of HCC, especially for AFP-negative patients.
The diagnosis of HCC can be made pathologically or via typical imaging hallmarks. For patients with liver cirrhosis or chronic hepatitis B/C, nodules >2 cm can be diagnosed as HCC based on the typical features of arterial phase hyper-enhancement (APHE). These include APHE with washout appearance at the portal venous or delayed or hepatobiliary phases on any of the three imaging modalities including multiphasic dynamic computed tomography (CT), dynamic magnetic resonance imaging (MRI), or gadolinium-ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced MRI (EOB-MRI). In addition, diagnosis can be based on the typical features of APHE with late (Ōēź60 seconds) washout appearance in the Kupffer phase on contrast-enhanced ultrasound (CEUS). On the other hand, the diagnosis of nodules Ōēż2 cm can be established when the typical features are present on at least two imaging modalities. Otherwise, a biopsy is recommended in case of an inconclusive diagnosis.
Nodules Ōēź1 cm can be diagnosed as HCC with typical hallmark on a single imaging technique in Japan [12], Korea [15], and western countries [16,17], whereas the diameter Ōēź2 cm is set as a prerequisite for the diagnosis via only one imaging modality in China. In fact, for nodules of 1ŌĆō2 cm, typical features on both dynamic CT and MRI used to be required for a definite diagnosis of HCC in western countries [18]. However, a decreased sensitivity with limited improvement in diagnostic specificity via coincidental dynamic CT plus MRI in later studies reaffirmed the application of a single modality to diagnose lesions Ōēź1 cm by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver (EASL) [19]. Considering the unbalanced distribution of medical resources in China, diagnostic accuracy is given priority over sensitivity. Therefore, confirmation via two imaging techniques for nodules of 1ŌĆō2 cm continues to be ad-opted in China. EOB-MRI and CEUS have been included as di-agnostic modalities to increase diagnostic sensitivity in China since 2017 [20].
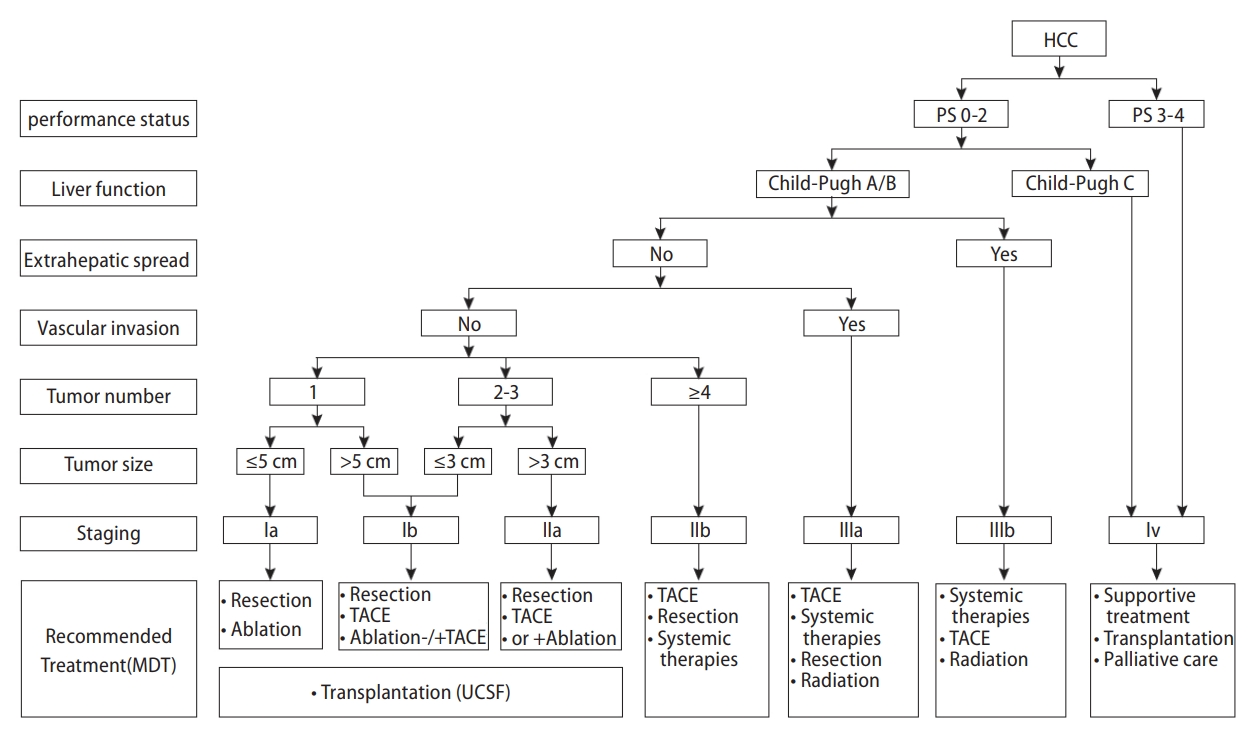
The China Liver Cancer Staging (CNLC) system (Fig. 1), which incorporates tumor characteristics, liver function, and performance status, similar to the Barcelona Clinic of Liver Cancer (BCLC) system [21], was established in 2017 and has been adopted ever since. Concerning tumor status, each stage of BCLC 0/A, B, and C is divided into two substages in the CNLC system, including stages Ia, Ib, IIa, IIb, IIIa, and IIIb. CNLC stage IV is equivalent to BCLC stage D.
Similar to the BCLC system, the CNLC system is a treatment allocation method for decision-making purposes, whereas the Japan Integrated Staging score [22] and its variants focus on the prognostic predictive function. The modified Union for International Cancer Control system [23] adopted in Korea is characterized by more detailed treatment allocation and is applied on the premise of the Child-Pugh A function, no portal hypertension, and performance score (PS) scoring 0ŌĆō1. The CNLC system has been in wide use in real-life practices, although the BCLC system continues to be the main stratification factor for clinical trial designing.
Hepatectomy is preferably indicated for patients with CNLC stage Ia, Ib, and IIa HCC. For patients with CNLC IIb and IIIa HCC who are optimal candidates for transarterial chemoembolization (TACE) and systemic therapy, respectively, surgical resection can be considered if tumor nodules are localized in the same segment or lobe [24], and tumor emboli are expected to be completely resected. Child-Pugh grade A, an indocyanine green (ICG) 15-minute retention rate <30%, and future remnant liver volume accounting for more than 40% (for patients with liver fibrosis/cirrhosis) or more than 30% (for patients without liver fibrosis/cirrhosis) are prerequisites for hepatectomy. Minimally invasive laparoscopic or robot-assisted laparoscopic liver resection (LLR) is recommended in experienced centers.
In real-life practice, conversion therapy via multimodal and high-intensity anti-tumor strategies is advocated to improve resectability and long-term survival for patients with potentially resectable HCC, defined as technically unresectable CNLC stage Ia, Ib, IIa HCC, or technically resectable IIb, IIIa HCC [25]. Systemic therapies like tyrosine kinase inhibitors (TKIs) plus programmed death-1 (PD-1) inhibitors [26,27] and locoregional treatments like hepatic arterial infusion chemotherapy (HAIC) plus TACE [28] have been explored as conversion therapies to induce tumor shrinkage or downstaging. As effective methods to introduce liver regeneration for patients with unmet future liver reserve, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) were deemed to be superior to portal vein embolization in the faster introduction of liver regeneration and fewer risks of tumor progression [29,30]. For patients who failed to achieve sufficient hypertrophy after conventional ALPPS stage-1 due to severe fibrosis/cirrhosis, transcatheter arterial embolization-salvaged ALPPS (TAE-salvaged ALPPS) is a new strategy to increase the resectability of HCC [31].
Lesions that are less than 10 cm and located in Couinaud segments II, III, IVb, V, and VI without affecting the anatomy of the first and second hepatic hilus used to be the best candidates for LLR [9]. With the development of minimally invasive techniques, especially the use of ICG fluorescence [32], indications for LLR are expanded without strict restrictions on tumor size and tumor location in an experienced center in China. Although propensity score-matched (PSM) studies [33-35] have affirmed comparable oncologic outcomes between LLR and open resection, especially for early-stage HCC [33], multicenter, randomized, controlled studies are warranted to validate the equal long-term efficacy of LLR compared to open resection.
The University of California San Francisco criteria (solitary tumor Ōēż6.5 cm or Ōēż3 nodules Ōēż4.5 cm plus total tumor diameter Ōēż8 cm) continues to be advocated as the major criteria for liver transplantation (LT) in the latest Chinese guideline. For patients who are initially beyond the LT criteria, downstaging therapies via locoregional therapies are recommended to reduce tumor burden to be within the LT criteria. Early withdrawal of or no corticosteroid [36] and replacement of calcineurin inhibitors with mammalian target of rapamycin (mTOR) inhibitors [37] are recommended to prevent tumor recurrence after LT.
In real-life practices, a number of criteria have been adopted in different centers, including Shanghai Fudan criteria [38], West China criteria [39], and Sanya consensus with minor differences in tumor number and tumor size. Although transplant patients are usually excluded from immune checkpoint inhibitor therapy for fear of possible graft rejection, experience in our center showed that grafts without PD-L1 expression seemed to represent a useful marker for not developing graft-related immune-related adverse events [40], the result of which needs confirmation by more studies.
Consistent with previous versions of clinical guidelines, ablation as a curative approach is indicated for CNLC Ia and Ib HCC (single nodule Ōēż5 cm, 2ŌĆō3 nodules with each Ōēż3 cm). Radiofrequency ablation (RFA) and microwave ablation (MWA) are recommended equally without priority. For unresectable single HCC with a diameter of 3ŌĆō7 cm, ablation in combination with TACE is recommended [41].
In real-life practices in China, ablation is not only applied to early HCC, its combination with TACE has also been implemented in patients with intermediate HCC. After PSM, MWA plus TACE yielded superior progression free survival (PFS) and overall survival (OS) to TACE alone for BCLC stage B HCC [42,43]. Notably, the morphological criteria and treatment modality varied among different studies. Zhang et al. [42] included patients with 2ŌĆō5 tumor nodules with each Ōēż7 cm as a target population for MWA in combination with TACE, whereas Li et al. [43] enrolled patients with either single tumors Ōēż8 cm or 2ŌĆō5 tumors each Ōēż5 cm. The above studies adopted a sequential treatment of MWA after TACE at an interval of at least one month, another study proposed a concurrent treatment of TACE and MWA [44]. On the other hand, although the role of PD-1 inhibitors in the adjuvant setting is still under investigation, promising results in early-stage clinical trials have encouraged the administration of PD-1 blockade in addition to RFA in real-life practices. A retrospective study demonstrated that PD-1 inhibitors in addition to RFA for recurrent HCC resulted in a significantly improved 1-year recurrence-free survival (RFS) rate compared to RFA alone [45].
TACE has been mainly indicated for CNLC IIb, IIIa, and some IIIb HCC since the 2017 Chinese guidelines. For patients with CNLC Ia, Ib, and IIa HCC ineligible for curative treatments, TACE is recommended as an alternative. Conventional TACE (cTACE) was equally recommended with drug-eluting bead-TACE due to similar OS benefits [46]. Super-selective TACE with the assistance of Cone-Beam CT if necessary is recommended to guarantee the efficacy of TACE [47]. cTACE is also recommended in the adjuvant setting for patients with high recurrent risks including multiple lesions, evidence of tumor thrombus or tumor diameter >5 cm [48].
In real-life practices, the combination of TACE with other locoregional treatment or systemic therapy is emphasized. Optimizing the management of intermediate-stage HCC has been a research hotspot [49]. Although sorafenib in combination with TACE yielded a similar OS to TACE alone for intermediate HCC [50], the improved PFS provided by sorafenib and the prolonged OS rendered by lenvatinib supported the addition of TKIs to TACE. Triple therapy of TACE integrated with TKIs plus PD-1 inhibitor for intermediate HCC showed favorable efficacy in controlling tumor progression and provided an opportunity for resection [51]. On the other hand, when TACE is indicated for HCC patients with portal vein invasion or extrahepatic metastasis in case collateral compensation exists or extrahepatic tumor burden is limited, the addition of systemic therapy such as sorafenib [52], lenvatinib [53-56], or lenvatinib plus PD-1 antibody [57] is a routine practice in some institutions and demonstrated with more favorable tumor control than systemic therapy alone. Y90 transarterial radioembolization (TARE) has not been widely applied in China currently.
While HAIC using interferon, cisplatin, or low-dose 5-FU plus cisplatin regimen alone or in combination with sorafenib was not recommended for advanced HCC due to negative results in Japan, HAIC using FOLFOX (folinic acid, fluorouracil, and oxaliplatin) regimen developed in China was not only adopted as an alternative treatment for TACE-refractory or TACE-unsuitable patients [58] but also as a first-line treatment for advanced HCC due to prolonged median OS compared to sorafenib for advanced HCC [59-61]. For patients with large unresectable HCC (largest diameter Ōēź7 cm) without macrovascular invasion or extrahepatic spread, FOLFOX-HAIC also significantly improved OS compared to TACE [62]. Moreover, the strong antitumor efficacy for intrahepatic lesions enabled FOLFOX-HAIC as a conversion therapy to transform HCC from unresectable to resectable [63]. A recent study reported that 1ŌĆō2 cycles of FOLFOX-HAIC in the adjuvant setting significantly improved RFS for patients with microvascular invasion [64]. Although FOLFOX-HAIC has not been clearly recommended in the current guidelines, it is accepted as an effective locoregional treatment modality across all stages of HCC in China. Nonetheless, there still lacks a consensus on the treatment modality of FOLFOX-HAIC in different regimes of FOLFOX (oxaliplatin, 85 or 130 mg/m2 for 2 hours; leucovorin, 400 mg/m2; fluorouracil bolus 400 mg/m2, and 5-fluorouracil, 2,400 mg/m2 for 46 hours or 2,400 mg/m2 for 24 hours or 1,200 mg/m2 for 22 hours) were administered in different studies. Moreover, the addition of systemic therapies including target agents and immunotherapy to HAIC turned out to improve overall response rate [28], although the long-term effects on liver function and OS need to be clarified in the future.
Stereotactic body radiotherapy is indicated for patients with CNLC Ia and some Ib HCC who are ineligible for curative treatments or who are reluctant to receive invasive treatment. For patients with CNLC IIa or IIb HCC, external beam radiation therapy in combination with TACE can improve local tumor control. For patients with resectable CNLC IIIa HCC, external ablation can be performed to control tumor thrombus in the neoadjuvant or adjuvant setting to prolong OS [65].
In real-life practices, external beam radiotherapy is carried out with recommendations in the guidelines. As for internal radiation, Y90 TARE has not been applied widely in China. On the other hand, TACE in combination with 125I seed and stent implantation has been implemented for patients with type II tumor thrombus and demonstrates superior survival benefits to TACE alone [66].
Systemic therapy is mainly indicated for patients with advanced HCC, namely CNLC IIIa and IIIb patients. For patients who had CNLC IIb HCC ineligible for locoregional therapies or who are refractory to TACE, transition to systemic therapy is recommended and covered by Chinese national reimbursement. The current Chinese guidelines recommend atezolizumab plus bevacizumab (Atezo-Bev) [67], suntilimab plus bevacizumab analog (Byvasda) [68], lenvatinib, sorafenib, donafenib [69], and FOLFOX chemotherapy as the first-line treatment without priority. Apart from regorafenib, targeted agent apatinib and PD-1 inhibitors camrelizumab [70] and tislelizumab [71] have been added as second-line systemic therapies for patients who have progressed on sorafenib. Cabozantinib [72] and ramucirumab [73], which are approved as second-line treatments for selected patients in other countries, have not been marketed in China.
Since 2017, with the approval of new first-line and second-line agents, the treatment options for advanced HCC are more diverse than ever. Apart from PD-1/PD-L1 inhibitor plus vascular endothelial growth factor receptor (VEGFR) inhibitor including Atezo-Bev and suntilimab-Byvasda, PD-1/PD-L1 inhibitors plus TKIs have been in wide use in real-life practices in China. The RESCUE trial, evaluating the efficacy of camrelizumab plus apatinib versus sorafenib as first-line therapy, met the dual primary endpoint and showed significant improvements in OS (22.1 vs. 15.2 months, P<0.001) and PFS (5.6 vs. 3.7 months, P<0.001) in the combination arm [74]. Although lenvatinib plus pembrolizumab versus lenvatinib monotherapy failed to meet pre-specified statistical significance in OS (21.2 vs. 19.0 months, P=0.023) in the phase III LEAP-002 study [75], the clinically meaningful survival benefit still provided a rationale for its use as first-line treatment. Sorafenib [76] or lenvatinib [77-79] plus PD-1 inhibitor as first-line treatment and regorafenib plus PD-1 inhibitor [80,81] as second-line treatment have shown promising survival benefits with manageable toxicity in Chinese patients. Nevertheless, the insignificant OS improvement of cabozantinib plus atezolizumab versus sorafenib (15.4 vs. 15.5 months, P=0.440) in the COSMIC-312 study suggested that immunotherapy in addition to targeted agents did not guarantee synergistic efficacy in the clinic [82,83].
Although dual immunotherapies of durvalumab plus tremelimumab [84] in the first-line setting and nivolumab plus ipilimumab [85,86] in the second-line setting approved in western countries have not been included in current Chinese guidelines, their applications as post-line therapies after TKI failure, PD-1/PD-L1 monotherapy failure or TKI plus PD-1/PD-L1 combination therapy failure are adopted in real-life practice. Further evidence for dual immunotherapy as post-line therapy is needed. On the other hand, the current approved second-line agents indicated for patients who progressed on sorafenib can apply equally to those who progressed on lenvatinib. According to a retrospective study, lenvatinib-regorafenib sequential therapy yielded prolonged total OS (29.7 vs. 23.0 months, P=0.041) and post-regorafenib OS (15.9 vs. 11.7 months, P=0.045) compared to sorafenib-regorafenib sequential therapy [87]. With the advent of more effective treatments, the optimal sequence or a combination modality suitable for individual patients needs to be clarified by high-evidence trials.
To provide useful and accessible information for all clinicians, the Chinese guidelines on the management of HCC incorporated literature based on not only randomized clinical trials (RCT) but also observational studies of newly developed clinical practices with levels of evidence classified by revised Grading of Recommendations, Assessment, Development and Evaluation [88]. Novel findings on the surveillance, diagnosis, and treatment of HCC are elaborated in the appendix of updated guidelines for a deep understanding of new trends.
Considering the aggressive nature of HBV-associated HCC, multimodal treatments like the addition of immunotherapy-based systemic treatment to local therapies have been adopted in real-life practices in China. The development of original drugs, such as apatinib, suntilimab, camrelizumab, and tislelizumab, and biologically similar drugs, such as Byvasda, in China, enable patients to accept the aforementioned multimodal treatment with an affordable economic burden. Nevertheless, with regard to social cost-effectiveness, only treatment with high-grade evidence-based RCTs is covered by insurance, which somehow narrows the gap from the optimal guideline recommendations to real-life practices at the government level. Moreover, more than 20 phase III trials are in progress to identify the role of ICI-based therapies across all stages of HCC [89]. The release of these results in the next 5 years should lead to a consensus on the addition of immunotherapy-based systemic therapy to HCC at different stages.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (No. 82130077, 82090053 and 81961128025), Basic Research Project from the Science and Technology
Commission of Shanghai Municipality (Grants 21JC1410100, 21JC1401200, 20JC1418900).
FOOTNOTES
Abbreviations
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
aMAP
age-Male-ALBI-Platelets
CNLC
Chinese liver cancer
HCV
hepatitis C virus
NASH
non-alcoholic steatohepatitis
US
ultrasonography
AFP
alpha-fetoprotein
APHE
arterial phase hyper-enhancement
CT
computed tomography
MRI
magnetic resonance imaging
EOB-MRI
gadoxetic acid (Gd-EOB-DTPA) enhanced magnetic resonance imaging
CEUS
contrast-enhanced ultrasound
EASL
European Association for the Study of the Liver
BCLC
Barcelona Clinic of Liver Cancer
TACE
transarterial chemoembolization
ICG
indocyanine green
LLR
laparoscopic liver resection
TKIs
tyrosine kinase inhibitors
PD-1
programmed death-1
HAIC
hepatic arterial infusion chemotherapy
ALPPS
associating liver partition and portal vein ligation for staged hepatectomy
PSM
propensity score-matched
LT
liver transplantation
RFA
radiofrequency ablation
MWA
microwave ablation
OS
overall survival
RFS
recurrence-free survival
cTACE
conventional TACE
DEB-TACE
drug-eluting bead-TACE
TARE
transarterial radioembolization
mOS
median OS
SBRT
stereotactic body radiotherapy
RCT
randomized clinical trial
GRADE
Grading of Recommendations
REFERENCES
1. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-1158. Erratum in: Lancet 2020;396:26.
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-249.



3. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598-1606.



4. de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 2015;62:1190-1200.




5. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550-6557.


6. Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res 2018;30:571-579.



7. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.


8. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-2166.




9. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 2020;9:682-720.




10. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer 2018;7:235-260.




11. Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020;73:1368-1378.

12. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019;49:1109-1113.



13. Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol 2011;29:4781-4788.


14. Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728-735.e4.


15. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705.


16. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817.
17. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-750.



18. Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48 Suppl 1:S20-37.


19. Aub├® C, Oberti F, Lonjon J, Pageaux G, Seror O, NŌĆÖKontchou G, et al.; CHIC Group. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int 2017;37:1515-1525.


20. Park J, Lee JM, Kim TH, Yoon JH. Imaging diagnosis of hepatocellular carcinoma: Future directions with special emphasis on hepatobiliary magnetic resonance imaging and contrast-enhanced ultrasound. Clin Mol Hepatol 2022;28:362-379.


21. Reig M, Forner A, Rimola J, Ferrer-F├Ābrega J, Burrel M, Garcia-Criado ├ü, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-693.

22. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38:207-215.


23. Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 2002;24:395-403.

24. Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-88.

25. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al.; Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr 2022;11:227-252.

26. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and Anti-PD-1 antibody combinations. Liver Cancer 2021;10:320-329.


27. Xie D, Sun Q, Wang X, Zhou J, Fan J, Ren Z, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med 2021;9:652.

28. Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy 2021;13:1395-1405.

29. Li PP, Huang G, Jia NY, Pan ZY, Liu H, Yang Y, et al. Associating liver partition and portal vein ligation for staged hepatectomy versus sequential transarterial chemoembolization and portal vein embolization in staged hepatectomy for HBV-related hepatocellular carcinoma: a randomized comparative study. Hepatobiliary Surg Nutr 2022;11:38-51.

30. Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg 2020;271:534-541.

31. Peng Y, Wang Z, Qu X, Chen F, Sun H, Wang X, et al. Transcatheter arterial embolization-salvaged ALPPS, a novel ALPPS procedure especially for patients with hepatocellular carcinoma and severe fibrosis/cirrhosis. Hepatobiliary Surg Nutr 2022;11:504-514.



32. Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann Surg 2021;274:97-106.

33. Zhu P, Liao W, Zhang WG, Chen L, Shu C, Zhang ZW, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic or open liver resection for patients with BCLC stage 0-A hepatocellular carcinoma. Ann Surg 2022 Jan 25;doi: 10.1097/SLA.0000000000005380.

34. Chen JF, Fu XT, Gao Z, Shi YH, Tang Z, Liu WR, et al. Laparoscopic vs. open repeat hepatectomy for recurrent liver tumors: a propensity score-matched study and meta-analysis. Front Oncol 2021;11:646737.

35. Song DJ, Zhu K, Tan JP, Cai JB, Lv MZ, Hu J, et al. Perioperative and oncologic outcomes of laparoscopic versus open liver resection for combined hepatocellular-cholangiocarcinoma: a propensity score matching analysis. Surg Endosc 2022 Sep 8;doi: 10.1007/s00464-022-09579-y.



36. Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, et al. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl 2008;14:512-525.

37. Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:62-69.



38. Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 2009;135:1403-1412.



39. Li J, Yan LN, Yang J, Chen ZY, Li B, Zeng Y, et al. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009;15:4170-4176.



40. Shi GM, Wang J, Huang XW, Huang XY, He YF, Ji Y, et al. Graft programmed death ligand 1 expression as a marker for transplant rejection following anti-programmed death 1 immunotherapy for recurrent liver tumors. Liver Transpl 2021;27:444-449.



41. Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-432.


42. Zhang R, Shen L, Zhao L, Guan Z, Chen Q, Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol 2018;24:219-224.


43. Li HZ, Tan J, Tang T, An TZ, Li JX, Xiao YD. Chemoembolization plus microwave ablation vs chemoembolization alone in unresectable hepatocellular carcinoma beyond the milan criteria: a propensity scoring matching study. J Hepatocell Carcinoma 2021;8:1311-1322.




44. Si ZM, Wang GZ, Qian S, Qu XD, Yan ZP, Liu R, et al. Combination therapies in the management of large (Ōēź 5 cm) hepatocellular carcinoma: microwave ablation immediately followed by transarterial chemoembolization. J Vasc Interv Radiol 2016;27:1577-1583.


45. Wang X, Liu G, Chen S, Bi H, Xia F, Feng K, et al. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: a propensity score matching analysis. Int J Hyperthermia 2021;38:1519-1528.


46. Liang B, Makamure J, Shu S, Zhang L, Sun T, Zheng C. Treatment response, survival, and safety of transarterial chemoembolization with CalliSpheres® microspheres versus conventional transarterial chemoembolization in hepatocellular carcinoma: a meta-analysis. Front Oncol 2021;11:576232.



47. Pung L, Ahmad M, Mueller K, Rosenberg J, Stave C, Hwang GL, et al. The role of Cone-Beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol 2017;28:334-341.


48. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res 2018;24:2074-2081.



49. Torimura T, Iwamoto H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin Mol Hepatol 2021;27:236-245.




50. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Final results of TACTICS: a randomized, prospective trial comparing transarterial chemoembolization plus sorafenib to transarterial chemoembolization alone in patients with unresectable hepatocellular carcinoma. Liver Cancer 2022;11:354-367.




51. Li J, Shi J, Song J. Efficacy and safety of transcatheter arterial chemoembolization in combination with camrelizumab and targeted agents in intermediate-stage unresectable hepatocellular carcinoma: A single-arm, prospective, real-world study. J Clin Oncol 2022;40(16 suppl):e16190.

52. Yuan J, Yin X, Tang B, Ma H, Zhang L, Li L, et al. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Biomed Res Int 2019;2019:2141859.




53. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol 2023;41:117-127.


54. Xia D, Bai W, Wang E, Li J, Chen X, Wang Z, et al. Lenvatinib with or without concurrent drug-eluting beads transarterial chemoembolization in patients with unresectable, advanced hepatocellular carcinoma: a real-world, multicenter, retrospective study. Liver Cancer 2022;11:368-382.




55. Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer 2021;127:3782-3793.



56. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int 2021;15:663-675.




57. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with Anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma 2021;8:1233-1240.




58. Hsu SJ, Xu X, Chen MP, Zhao ZY, Wang Y, Yin X, et al. Hepatic arterial infusion chemotherapy with modified FOLFOX as an alternative treatment option in advanced hepatocellular carcinoma patients with failed or unsuitability for transarterial chemoembolization. Acad Radiol 2021;28 Suppl 1:S157-S166.


59. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 2018;69:60-69.


60. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol 2019;5:953-960.



61. Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol 2022;40:468-480.


62. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol 2022;40:150-160.


63. Wang J, Zheng Z, Wu T, Li W, Wang J, Pan Y, et al. Hepatic arterial infusion chemotherapy as a timing strategy for conversion surgery to treat hepatocellular carcinoma: a single-center real-world study. J Hepatocell Carcinoma 2022;9:999-1010.




64. Li S, Mei J, Cheng Y, Li Q, Wang Q, Fang C, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy (HAIC) with FOLFOX to improve outcomes of patients with hepatocellular carcinoma with microvascular invasion: A prospective multicenter, phase 3, randomized, controlled clinical trial. J Clin Oncol 2022;40(16 suppl):4013.

65. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141-2151.



66. Zhang ZH, Zhang W, Gu JY, Liu QX, Ma JQ, Liu LX, et al. Treatment of hepatocellular carcinoma with tumor thrombus with the use of iodine-125 seed strand implantation and transarterial chemoembolization: a propensity-score analysis. J Vasc Interv Radiol 2018;29:1085-1093.

67. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al.; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.


68. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al.; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-990. Erratum in: Lancet Oncol 2021;22:e347.
69. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallelcontrolled phase II-III trial. J Clin Oncol 2021;39:3002-3011.



70. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol 2022;33 Suppl 7:S1401-S1402.
71. Qin S, Kudo M, Meyer T, Finn RS, Vogel A, Bai Y, et al. Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol 2022;33 Suppl 7:S1402-S1403.
72. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54-63.



73. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al.; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased ╬▒-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-296.


74. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-580.


75. Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 2022;33 Suppl 7:S1401.
76. Zeng Z, Zhang L, Wu T, Cheng J, Chen Y, Lu Y. A retrospective study on the efficacy and safety of sorafenib or lenvatinib combined with sintilimab in patients with advanced hepatocellular carcinoma. J Clin Oncol 2021;39(15 suppl):e16127.

77. Chang X, Yu S, Pang J, Zhang W, Kong H, Huang J, et al. The best benefit population treated with lenvatinib plus anti-PD-1 for unresectable hepatocellular carcinoma. J Clin Oncol 2022;40(16 suppl):e16152.

78. Yang X, Chen B, Wang Y, Wang Y, Long J, Zhang N, et al. Lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma: A large real-world study from two centers. J Clin Oncol 2022;40(16 suppl):e16155.

79. Sun H-C, Zhu X-D, Huang C, Shen Y-H, Ge N-L, Chen Y, et al. Combination therapy with lenvatinib and anti-PD-1 antibodies for unresectable or advanced hepatocellular carcinoma: A real-world study. J Clin Oncol 2020;38(15 suppl):e16610.

80. Li Z, Li L, Zhong J, Cui K, Zhang C, Shi X, et al. Regorafenib combined with PD-1 monoclonal antibody in the second-line setting for hepatocellular carcinoma (HCC): A retrospective, real-world study in China. J Clin Oncol 2022;40(16 suppl):e16145.

81. Yan T, Peng C, Yu L, Duan X, Ji D, Zhang L, et al. A retrospective study on the efficacy and safety of regorafenib or regorafenib combined with immune-checkpoint-inhibitors (ICIs) after first-line therapy in patients with advanced hepatocellular carcinoma. J Clin Oncol 2022;40(16 suppl):e16115.

82. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008.


83. Cabibbo G, Celsa C, DŌĆÖAlessio A, Fulgenzi CAM, Pinato DJ. COSMIC-312: mounting immunotherapy enigmas for hepatocellular carcinoma. Lancet Oncol 2022;23:e441.


84. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol 2022;40(4 suppl):379.

85. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564. Erratum in: JAMA Oncol 2021;7:140.
86. El-Khoueiry AB, Yau T, Kang Y-K, Kim T-Y, Santoro A, Sangro B, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J Clin Oncol 2021;39(3 suppl):269.


87. Xue F, Zhai J, Liu J, Fu Z, Sun Y, Ge R, et al. Efficacy and safety of second-line regorafenib after sorafenib or lenvatinib first line in patients with unresectable hepatocellular carcinoma: A real-world study. J Clin Oncol 2022;40(16 suppl):e16125.

-
METRICS

- ORCID iDs
-
Qiang Gao

https://orcid.org/0000-0002-6695-9906 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



