| Clin Mol Hepatol > Volume 29(2); 2023 > Article |
|
ABSTRACT
Striking advances in systemic therapy for unresectable advanced hepatocellular carcinoma (HCC) have improved the average prognosis of patients with HCC. As a result, the guidelines for the treatment of HCC have changed significantly. However, various issues have emerged in clinical practice. First, there is no established biomarker that can predict response to systemic therapy. Second, there is no established treatment regimen after primary systemic therapy, including combined immunotherapy. Third, there is no established treatment regimen for intermediate-stage HCC. These points make the current guidelines ambiguous. In this review, we present the Japanese guidelines for the diagnosis and treatment of HCC based on the latest evidence; introduce various efforts mainly in Japanese real-life practice to update these guidelines; and present our perspectives on future guidelines.
Primary liver cancer is the fifth most common cause of death in Japan [1] and remains a serious disease in the national healthcare system. The number of new patients with hepatitis C virus (HCV)-related liver cancer, which previously accounted for 80% of all liver cancers in Japan, has continued to decline [2,3]. Currently, it accounts for approximately 40% of all cases. However, prevalence of non-viral liver cancers is increasing. An efficient surveillance system for this type of liver cancer, occurring in association with metabolic disorders such as nonalcoholic fatty liver diseases [4], diabetes mellitus, and alcohol overdose, has not been developed. It is especially challenging when the liver cancer is already advanced at the time of diagnosis in patients with such metabolic disorders.
Recently, a paradigm change in the systemic treatment of advanced hepatocellular carcinoma (HCC) has occurred globally. In addition to multikinase inhibitors such as sorafenib and lenvatinib, combined immunotherapy such as atezolizumab plus bevacizumab is now widely used in clinical practice as a first-line systemic therapy, with real-world reports of improved prognosis [5,6]. Furthermore, another combination therapy with immune checkpoint inhibitors (ICIs) will soon be covered by health insurance [7]. However, issues such as the establishment of biomarkers to predict response and how to proceed with conversion therapy combined with locoregional therapies remain unaddressed. In this article, we will review the current status of the clinical management of HCC in Japan, including recent findings and future trends based on the Guidelines for Liver Cancer Treatment 2021 published by the Japan Society of Hepatology (JSH) [8].
Patients with any of the following conditions, cirrhosis, chronic hepatitis B, or chronic hepatitis C, are considered to be at high risk for HCC, and those with cirrhosis type B (hepatitis B surface antigen-positive) and C (anti-HCV antibodypositive) are considered to be extremely high-risk groups for HCC. Ultrasonography (US) is considered a preferred surveillance modality with simultaneous measurements of alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), and the AFP-L3 fraction (a lectin-reactive fraction of AFP). US surveillance should be performed every six months in high-risk patients and every 3ŌĆō4 months in extremely high-risk patients in Japan [8].
Dynamic computed tomography (CT) or dynamic magnetic resonance imaging (MRI), including gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI, can be combined with US surveillance in extremely high-risk patients and/or in patients whose livers are difficult to scan using US due to liver atrophy, severe obesity, and post-operative deformity. When nodular lesions are detected by US, CT/MRI is performed for differential diagnosis. Even when a tumor is not detected via US, dynamic CT/MRI should still be considered in the following cases: persistent elevation of AFP, AFP Ōēź200 ng/mL, DCP Ōēź40 mAU/mL, or AFP-L3 fraction Ōēź15%. Tumor evaluation using Gd-EOB-DTPA-enhanced MRI or other diagnostic modalities, including liver biopsy, contrast-enhanced US, superparamagnetic iron oxideenhanced MRI, or CT during arterial portography or hepatic arteriography, is performed for tumors larger than 1.5 cm in diameter with negative arterial enhancement and for tumors larger than 1 cm in diameter with positive arterial enhancement and negative delayed washout. Other smaller lesions are followed up with US every three months. Dynamic CT/MRI should be resumed when tumor enlargement or elevated tumor marker levels are observed. Lesions not visualized on US may be followed up with dynamic CT/MRI [8].
A major feature of the Japanese surveillance system is the use of the tumor markers AFP-L3 fraction and DCP. The assessment of these markers is covered under public health insurance. The addition of AFP-L3 assessment to US+AFP assessment is known to improve sensitivity in the diagnosis of HCC [9] and is routinely used for surveillance extremely high-risk patients with chronic liver diseases. For such patients, simultaneous measurement of the tumor markers, AFP, AFP-L3, and DCP, is allowed under the health insurance system in Japan. Currently, its cost is 2,900 yen (approximately 22 dollars). Considering that the cost for independently measuring AFP, AFP-L3, and DCP is 1,010, 1,900, and 1,350 yen, respectively, amounting to 4,260 yen (approximately 32 dollars), it can be inferred that simultaneous measurement of the three markers helps to suppress over-measurement of tumor markers by medical institutions and to reduce expenditures from the insurance fund. Regarding MRI, most hepatologists in Japan prefer to use Gd-EOB-DTPA-enhanced MRI over conventional dynamic MRI with extracellular contrast agents. Recently, it has been suggested that Gd-EOB-DTPA-enhanced MRI may play an important role in predicting Wnt/╬▓-catenin signal-activated HCC, which is considered to have an ŌĆ£immune cold microenvironmentŌĆØ and is primarily resistant to treatment with immune checkpoint inhibitors alone [10].
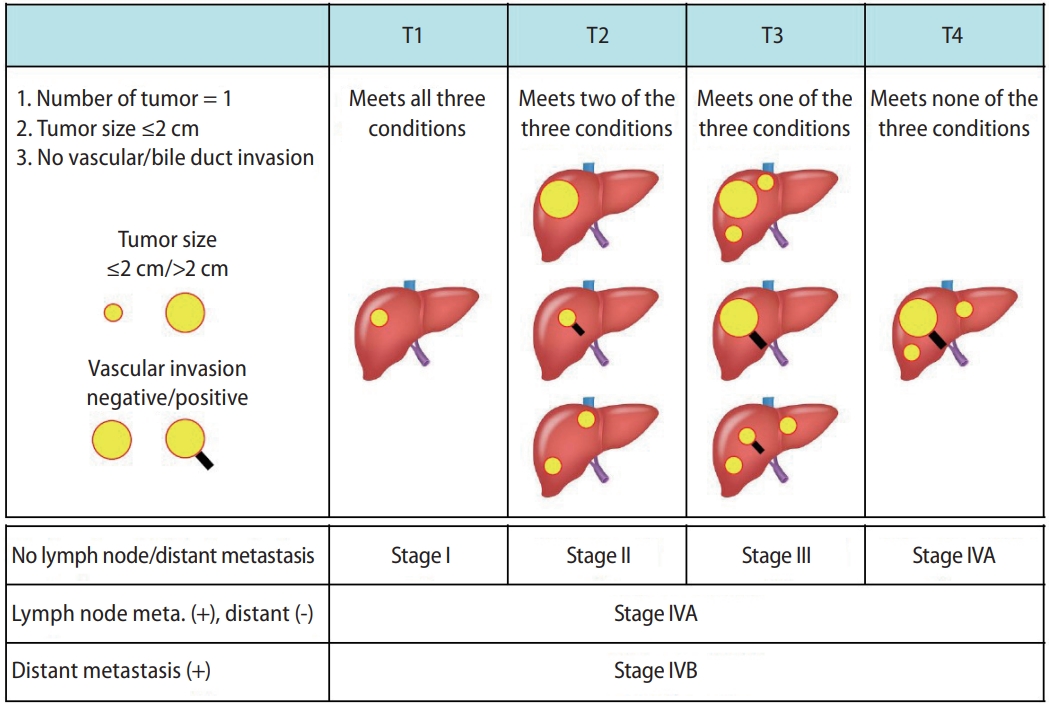
The tumor staging systems for HCC in Japan include the modified Union for International Cancer Control (mUICC) staging system [11] and the General Rules for the Clinical and Pathological Study of Primary Liver Cancer established by the Japan Liver Cancer Association (Fig. 1) [12]. The latter has been used for a nationwide follow-up survey of HCC in Japan, and the data is updated biannually.
From a therapeutic point of view, the Barcelona Clinic Liver Cancer (BCLC) staging system is the most popularly used system in Japan and other countries. The system is useful for determining therapeutic options for HCC based on clinical information, including performance status, hepatic functional reserve, and tumor characteristics.
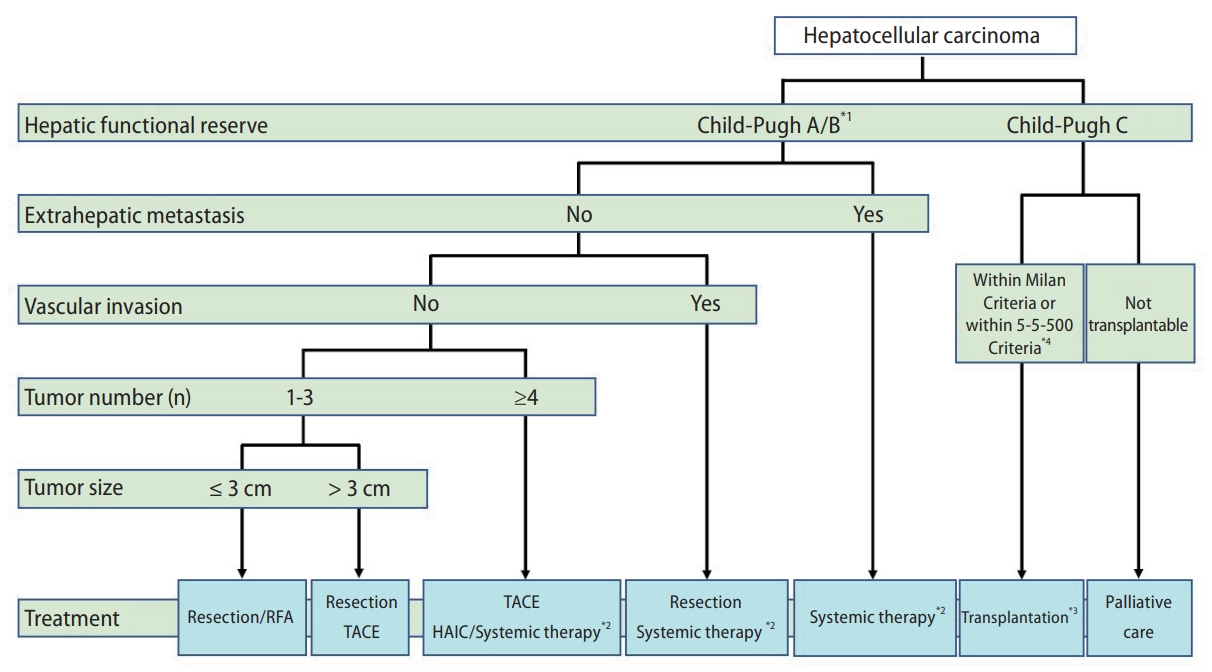
According to the Guidelines for Liver Cancer Treatment 2021, the treatment algorithm for HCC is based on five factors: hepatic functional reserve, extrahepatic metastasis, vascular invasion, tumor number, and tumor size (Fig. 2) [8]. For patients with Child-Pugh class A or B, the following three treatment options are recommended in the absence of extrahepatic metastases and vascular invasion: 1) If the number of tumors is 1ŌĆō3 and the tumor diameter is <3 cm, hepatic resection or radiofrequency ablation (RFA) is recommended. If one tumor is present, hepatic resection is recommended as the first choice, regardless of the tumor diameter. 2) If the number of tumors is 1ŌĆō3 and the tumor diameter is greater than 3 cm, hepatic resection is recommended as the first choice, followed by transcatheter arterial chemoembolization/embolization (TACE/TAE) as the second choice. 3) If the number of tumors is four or more, TACE is recommended as the first choice, followed by hepatic arterial infusion chemotherapy (HAIC) or systemic drug therapies, using molecular-targeted agents (MTAs) and ICIs as the second choice. Systemic drug therapy is recommended for patients with HCC, Child-Pugh class A cirrhosis, and extrahepatic metastases [8]. Liver transplantation (LT) is recommended for HCC patients with Child-Pugh class C cirrhosis if they are within the Milan criteria (three or fewer tumors and Ōēż3 cm in diameter, or one tumor Ōēż5 cm in diameter) or 5-5-500 criteria (five or fewer tumors, Ōēż5 cm in diameter, and AFP Ōēż500 ng/mL) and the patient is under 65 years of age. These criteria for liver transplantation are different from those used in the BCLC staging system. Palliative care is recommended for HCC patients with Child-Pugh class C cirrhosis who are unsuitable for LT [8].
Hepatic resection, LT, and locoregional therapy (ethanol injection, microwave coagulo-necrotic therapy, and RFA) for HCC are considered to be curative treatments in Japan [13,14]. According to Kudo et al. [15], 40.3% of patients were treated with hepatic resection or LT and 21.1% of patients were initially treated with locoregional therapy. The median overall survival (OS) of patients who underwent hepatic resection was 92.5 months, and the 5- and 10-year survival rates were 66.7% and 40.8%, respectively. Conversely, the median OS of patients who underwent RFA was 75.8 months, and the 5- and 10-year survival rates were 61.7% and 28.0%, respectively [15]. According to the results of the SURF trial [16], a domestic randomized controlled trial that compared the OS following hepatic resection and that following RFA, RFA is now considered equally effective to hepatic resection. In this trial, recurrence-free survival (RFS) did not differ significantly between groups. The median RFS was 3.5 years in the hepatic resection group and 3.0 years in the RFA group (hazard ratio [HR], 0.92; 95% confidence interval [CI], 0.67ŌĆō1.25; P=0.58). Thus, RFS did not differ significantly following hepatic resection or RFA in patients with the largest HCC diameter Ōēż3 cm and Ōēż3 HCC nodules.
In addition to intrahepatic HCC lesions, RFA also appears to be effective in patients with pulmonary HCC metastases [17].
TACE is the standard of care for patients with intermediatestage HCC and has been used worldwide [18-20]. Balloon-occluded TACE (B-TACE), a type of TACE, was established in Japan by Irie et al. [21]. Recently, it has been reported that substantially longer local recurrence-free periods were observed after B-TACE than after conventional TACE and other types of TACE [22]. However, repeated TACE is associated with a high rate of treatment failure, worsening liver function, and poor prognosis [23,24]. Therefore, the concept of unsuitable TACE for intermediate-stage HCC was recently proposed. Unsuitable TACE is generally defined as follows: 1) likely to develop TACE failure/refractoriness, 2) likely to develop Child-Pugh class B liver function after TACE, and 3) unlikely to respond to TACE. Unsuitable TACE includes patients, who exceed the up-to-seven criteria [25,26], those, who have liver function classified as modified albumin-bilirubin (mALBI) grade 2b, or those, who have HCC other than the simple nodular type.
Recently, several studies have reported that combining MTA and TACE therapy significantly improved OS compared to that associated with TACE alone in patients with unresectable HCC [27-29]. The TACE therapy in combination with sorafenib (TACTICS) trial showed that sorafenib-TACE sequential therapy yielded significantly longer progression-free survival (PFS) compared to that provided by TACE alone (25.2 months vs. 13.5 months, HR, 0.59; 95% CI, 0.41ŌĆō0.87; P<0.0001) [28]. Moreover, Kudo et al. [27] reported the beneficial effects of lenvatinib on the OS rate in patients with intermediate-stage HCC showing large or multinodular tumors exceeding the up-to-seven criteria. To improve survival in patients with intermediate-stage HCC unsuitable for TACE, this strategy was approved at a consensus meeting of the Asia-Pacific Primary Liver Cancer Expert (APPLE) Association [30] and the JSH. Furthermore, upfront systemic therapy was also recently recommended for patients who are TACE-unsuitable in the European Society for Medical Oncology clinical practice guidelines and the American Association for the Study of Liver Diseases [31,32].
HAIC was the standard therapy for advanced HCC in Japan [33,34]. However, the role of HAIC has been reconsidered due to recent progress in systemic therapies [35]. Combination therapies of HAIC with systemic therapies have recently attracted attention. Ikeda et al. [36] reported the effectiveness of combining cisplatin HAIC monotherapy with sorafenib in a randomized phase 2 clinical trial. Kudo et al. [37] reported a randomized phase 3 clinical trial comparing sorafenib monotherapy with sorafenib plus a low-dose cisplatin/5-fluorouracil (FP) HAIC regimen. In that study, low-dose FP plus sorafenib did not show any additive survival benefits in all enrolled patients, compared to that with sorafenib alone. However, the combination therapy was significantly more effective in patients with advanced HCC with severe portal vein tumor thrombus. Combination therapy using HAIC and lenvatinib has also been reported. Shimose et al. [29] reported the effectiveness of combination therapy using a New FP HAIC regimen (Lipiodol-suspended FP) and lenvatinib. Their study revealed that alternating therapy with the New FP regimen and lenvatinib significantly prolonged the administration period of lenvatinib and patient survival. Sequential therapy from HAIC to systemic therapy or systemic therapy to HAIC is also challenging. Kondo et al. [38] reported a clinical trial of sequential HAIC and sorafenib treatment. However, the trial showed that this treatment did not improve the survival benefits compared to that with sorafenib alone. Therefore, the establishment of multidisciplinary therapeutic strategies for the management of advanced HCC remains an unmet medical need in Japan.
Since April 2022, particle therapies using protons and carbon ions have been applied to large (Ōēź4 cm) and difficult-toresect HCCs and are covered by health insurance in Japan [39]. Further studies are needed to establish solid evidence for both stereotactic body radiation therapy and particle therapy [39,40].
Compared to those in lenvatinib therapy, atezolizumab plus bevacizumab therapy prolonged PFS preserved hepatic functional reserve and resulted in lower rates of severe adverse effects (AEs). Thus, combination therapy is often selected as the first-line treatment, except for patients who should avoid immunotherapies or those with impaired liver function [41-44]. Kudo [45] recently advocated the concept of ŌĆśABC conversionŌĆÖ with the aim of a cancer-free/treatment-free status. This concept proposes using atezolizumab plus bevacizumab followed by curative conversion for patients with advanced HCC. The conversion rate in an atezolizumab plus bevaci zumab treatment group was reported to be higher than that in a lenvatinib treatment group (8.6% vs. 1.9%, P=0.007) [43], and resulted in a high conversion rate (35% [38/110]) with 22% of cases achieving a cancer-free/treatment-free status after receiving atezolizumab plus bevacizumab treatment [46]. To determine the true therapeutic effects of atezolizumab plus bevacizumab treatment, it is important to understand the discrepancy between radiological findings and biochemical responses [47].
Lenvatinib is also used as a front-line treatment because of the accumulated clinical evidence and innovations, such as the weekends-off method [48], which is an attempt to reduce AEs while maintaining therapeutic efficacy. Consequently, 66.7% of patients who were intolerant to prior lenvatinib therapy completed the weekends-off strategy with an improved therapeutic response in 61.5% of those patients. For patients with lenvatinib-refractory HCC, sorafenib is a possible treatment option [49]. Fortunately, lenvatinib-sorafenib sequential therapy is available in Japan under the insurance system. However, it is difficult to switch to sorafenib in patients who have discontinued lenvatinib due to AEs such as palmar-planter erythrodysesthesia. Further studies are needed to optimize sequential systemic drug therapy for lenvatinib-refractory HCC. Furthermore, combining lenvatinib with TACE to enhance antitumor effects [50] has a significant survival benefit, particularly in patients with non-viral HCC [51]. Additionally, in the final analysis for the phase II trial (TACTICS-L trial), the combination of TACE and lenvatinib showed promising therapeutic efficacy in patients with unresectable HCC [52].
Although there is no significant evidence for second-line treatment in patients in whom atezolizumab plus bevacizumab has failed, ramucirumab [53,54] and lenvatinib [55] have shown promising results as second-line treatments. Cabozantinib may have beneficial effects in patients who have received one or two prior systemic anticancer therapies for advanced HCC with subsequent radiographic progression [56]. The determination of ideal sequential systemic chemotherapy, including atezolizumab plus bevacizumab, as front- or later-line treatment is complex and controversial; therefore, further evidence should be accumulated.
From a global perspective, there are two major trends in the clinical management of HCC. One is the futuristic challenges in diagnostics, including artificial intelligence (AI) [57], and the other is the further advancement of combined immunotherapy for advanced HCC. The introduction of AI for diagnosing HCC will not only improve diagnostic accuracy but also lead to accurate prediction of treatment efficacy in collaboration with multi-omics analysis [58]. Thus, AI will integrate various types of images and biological information and provide real-time information to determine whether the tumor immune microenvironment is hot or cold, determine the degree of responsiveness to MTAs, and predict endogenous genetic changes in the tumors in the near future. In Japan, results from exploratory research on AI diagnosis of HCC are emerging [59,60].
The emergence of combined immunotherapy for advanced HCC has brought about a paradigm change. However, in the absence of appropriate biomarkers to predict therapeutic efficacy, achieving a cure with systemic therapy alone is challenging. Although chimeric antigen T-cell therapies and other therapies are being developed [61], the most urgent need is identifying the optimal solution for second-line and later therapies using currently available drugs. In this regard, a prospective observational registry study called the PRISM study is underway in Japan to determine the optimal systemic therapy to follow atezolizumab plus bevacizumab therapy for unresectable HCC patients. The results of the PRISM study are expected to help manage the chaotic situation in determining the optimal second-line systemic treatment, according to the current guidelines in Japan.
FOOTNOTES
AuthorsŌĆÖ contribution
TK drew up the basic plan for writing this paper and HI proposed the role assignment. MN wrote the Curative treatment part, SS wrote the TACE part, HI wrote the HAIC part, HS wrote the Systemic therapies description, and HK wrote the ABSTRACT, INTRODUCTION, SURVEILLANCE AND DIAGNOSIS, STAGING, DISCUSSION, and TREATMENT guideline overview. HK integrated all described parts and refined the article. All authors participated in a critical discussion.
Conflicts of Interest
H.K. received lecture fees from Chugai Pharmaceutical Co. Ltd. and Eisai Co. Ltd. T.K. received lecture fees from Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co. Ltd., Kowa Company Ltd., Otsuka Pharmaceutical Co. Ltd., Eisai Co. Ltd., ASKA Pharmaceutical Co. Ltd., and AbbVie GK. T.K. received research funding from Eisai Co. Ltd. The other authors have no conflicts of interest pertaining to this study.
Figure┬Ā1.
The staging system for HCC according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer (the Japan Liver Cancer Association). HCC, hepatocellular carcinoma; Meta, metastasis.
Figure┬Ā2.
The treatment algorithm for HCC according to the Guidelines for Liver Cancer Treatment 2021 in Japan. The algorithm is based on five factors: hepatic functional reserve, extrahepatic metastasis, vascular invasion, tumor number, and tumor size. *1. Assessment based on liver damage is recommended in the case of hepatectomy; *2. Patients with ChildŌĆōPugh class A only; *3. Patients aged Ōēż65 years; and *4. No extrahepatic metastasis or vascular invasion. Five or fewer tumors, size Ōēż5 cm in diameter, and AFP Ōēż500 ng/mL. HCC, hepatocellular carcinoma; HAIC, hepatic arterial infusion chemotherapy; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization; AFP, alpha-fetoprotein.
Abbreviations
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
JSH
Japan Society of Hepatology
US
ultrasonography
AFP
alpha-fetoprotein
DCP
des-gamma-carboxy prothrombin
CT
computed tomography
MRI
magnetic resonance imaging
Gd-EOB-DTPA
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid
mUICC
modified Union for International Cancer Control
BCLC
Barcelona Clinic Liver Cancer
RFA
radiofrequency ablation
TACE
transcatheter arterial chemoembolization
TAE
transcatheter arterial embolization
HAIC
hepatic arterial infusion chemotherapy
MTAs
molecular-targeted agents
ICIs
immune checkpoint inhibitors
OS
overall survival
RFS
recurrence-free survival
B-TACE
Balloon-occluded TACE
mALBI
modified albumin-bilirubin
TACTICS
TACE therapy in combination with sorafenib
PFS
progression-free survival
APPLE
Asia-Pacific Primary Liver Cancer Expert
AEs
adverse effects
AI
artificial intelligence
REFERENCES
1. Summary of Vital Statistics. Number and rate of death by sex, by simple cause of death classification (per 100,000 population): Ministry of Health, Labour and Welfare of Japan. 2020.
2. Tateishi R, Okanoue T, Fujiwara N, Okita K, Kiyosawa K, Omata M, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: A large retrospective multicenter cohort study. J Gastroenterol 2015;50:350-360.




3. Nakano M, Yatsuhashi H, Bekki S, Takami Y, Tanaka Y, Yoshimaru Y, et al. Trends in hepatocellular carcinoma incident cases in Japan between 1996 and 2019. Sci Rep 2022;12:1517.




4. Kawaguchi T, Tsutsumi T, Nakano D, Eslam M, George J, Torimura T. MAFLD enhances clinical practice for liver disease in the Asia-Pacific region. Clin Mol Hepatol 2022;28:150-163.




5. Rimini M, Persano M, Tada T, Suda G, Shimose S, Kudo M, et al. Real-world data for atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: How does adherence to the imbrave150 trial inclusion criteria impact prognosis? Target Oncol 2023 Mar 15;doi: 10.1007/s11523-023-00953-x.
6. Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, et al.; Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Does first-line treatment have prognostic impact for unresectable HCC?-Atezolizumab plus bevacizumab versus lenvatinib. Cancer Med 2023;12:325-334.

7. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al.; HIMALAYA Investigators. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 2022;1(8):doi: 10.1056/EVIDoa2100070.

8. The Japan Society of Hepatology. Clinical Practice Guidelines for Hepatocellular Carcinoma 2021 Version: JSH HCC Guidelines 2021. KANEHARA & Co., LT.
9. Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology 2019;69:1983-1994.


10. Kudo M. Gd-EOB-DTPA-MRI could predict WNT/╬▓-catenin mutation and resistance to immune checkpoint inhibitor therapy in hepatocellular carcinoma. Liver Cancer 2020;9:479-490.




11. Brierley JD, Gospodarowicz MK, Christian W. TNM classification of malignant tumours. 8th ed. Hoboken: Wiley Blackwell; 2016.
12. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: The outstanding achievements of the liver cancer study group of Japan. Dig Dis 2015;33:765-770.



13. Taketomi A. Hepatic resection for hepatocellular carcinoma in the era of molecular-targeted agents and immune checkpoint inhibitors in Japan. JMA J 2021;4:241-245.



14. Tada T, Kumada T, Toyoda H, Tsuji K, Hiraoka A, Itobayashi E, et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci 2017;108:1414-1420.




15. Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 22nd nationwide follow-up survey of primary liver cancer in Japan (2012-2013). Hepatol Res 2022;52:5-66.



16. Takayama T, Hasegawa K, Izumi N, Kudo M, Shimada M, Yamanaka N, et al. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: A randomized controlled trial (SURF Trial). Liver Cancer 2021;11:209-218.




17. Hiraki T, Yamakado K, Ikeda O, Matsuoka T, Kaminou T, Yamagami T, et al. Percutaneous radiofrequency ablation for pulmonary metastases from hepatocellular carcinoma: Results of a multicenter study in Japan. J Vasc Interv Radiol 2011;22:741-748.


18. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817.
19. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705.




20. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019;49:1109-1113.



21. Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: Measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol 2013;36:706-713.



22. Shirono T, Iwamoto H, Niizeki T, Shimose S, Kajiwara A, Suzuki H, et al. Durable complete response is achieved by balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Commun 2022;6:2594-2604.




23. Hiraoka A, Kumada T, Kudo M, Hirooka M, Koizumi Y, Hiasa Y, et al.; Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics). Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: Multicenter analysis. Dig Dis 2017;35:602-610.



24. Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: Upfront systemic therapy followed by curative conversion. Liver Cancer 2021;10:539-544.




25. Arizumi T, Minami T, Chishina H, Kono M, Takita M, Yada N, et al. Time to transcatheter arterial chemoembolization refractoriness in patients with hepatocellular carcinoma in kinki criteria stages B1 and B2. Dig Dis 2017;35:589-597.



26. Shimose S, Kawaguchi T, Iwamoto H, Niizeki T, Shirono T, Tanaka M, et al. Indication of suitable transarterial chemoembolization and multikinase inhibitors for intermediate stage hepatocellular carcinoma. Oncol Lett 2020;19:2667-2676.



27. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh a liver function: A proof-of-concept study. Cancers (Basel) 2019;11:1084.



28. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al.; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-1501.



29. Shimose S, Iwamoto H, Tanaka M, Niizeki T, Shirono T, Noda Y, et al. Alternating lenvatinib and trans-arterial therapy prolongs overall survival in patients with inter-mediate stage hepatocellular carcinoma: A propensity score matching study. Cancers (Basel) 2021;13:160.



30. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020;9:245-260.




31. Vogel A, Martinelli E; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-805.


32. Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al.; AASLD Panel of Experts on Trial Design in HCC. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021;73 Suppl 1:158-191.



33. Moriya K, Namisaki T, Sato S, Furukawa M, Douhara A, Kawaratani H, et al. Bi-monthly hepatic arterial infusion chemotherapy as a novel strategy for advanced hepatocellular carcinoma in decompensated cirrhotic patients. Clin Mol Hepatol 2019;25:381-389.




34. Ueshima K, Komemushi A, Aramaki T, Iwamoto H, Obi S, Sato Y, et al. Clinical practice guidelines for hepatic arterial infusion chemotherapy with a port system proposed by the Japanese Society of Interventional Radiology and Japanese Society of Implantable Port Assisted Treatment. Liver Cancer 2022;11:407-425.




35. Iwamoto H, Shimose S, Shirono T, Niizeki T, Kawaguchi T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in the era of chemo-diversity. Clin Mol Hepatol 2023 Feb 13;doi: 10.3350/cmh.2022.0391.


36. Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann Oncol 2016;27:2090-2096.



37. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al.; SILIUS study group. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol 2018;3:424-432.


38. Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer 2019;19:954.




39. Shibuya K, Katoh H, Koyama Y, Shiba S, Okamoto M, Okazaki S, et al. Efficacy and safety of 4 fractions of carbon-ion radiation therapy for hepatocellular carcinoma: A Prospective Study. Liver Cancer 2021;11:61-74.




40. Kimura T, Fujiwara T, Kameoka T, Adachi Y, Kariya S. The current role of stereotactic body radiation therapy (SBRT) in hepatocellular carcinoma (HCC). Cancers (Basel) 2022;14:4383.

41. Tanaka T, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, et al.; Real-life Practice Experts for HCC (RELPEC) Study Group; HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol Res 2022;52:773-783.



42. Maesaka K, Sakamori R, Yamada R, Doi A, Tahata Y, Miyazaki M, et al. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res 2022;52:630-640.



43. Niizeki T, Tokunaga T, Takami Y, Wada Y, Harada M, Shibata M, et al. Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: A propensity score matching analysis. Target Oncol 2022;17:643-653.




44. Casadei-Gardini A, Rimini M, Tada T, Suda G, Shimose S, Kudo M, et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: A large real-life worldwide population. Eur J Cancer 2023;180:9-20.
45. Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC Conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer 2022;11:399-406.




46. Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Hagiwara S, et al. Achievement of cancer- and treatment-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: A multicenter cohort study. J Clin Oncol 2023;41 Suppl:Abstract no. 535.

47. Iwamoto H, Shimose S, Niizeki T, Koga H, Torimura T. Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma. Clin Mol Hepatol 2022;28:575-579.


48. Iwamoto H, Suzuki H, Shimose S, Niizeki T, Nakano M, Shirono T, et al. Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers (Basel) 2020;12:1010.

49. Kim Y, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, et al. Sorafenib versus nivolumab after lenvatinib treatment failure in patients with advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2023;35:191-197.


50. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol 2023;41:117-127.


51. Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open 2022;7:100591.


52. Ueshima K, Ishikawa T, Saeki T, Morimoto N, Aikata H, Tanabe N, et al. Transcatheter arterial chemoembolization therapy in combination strategy with lenvatinib in patients with unresectable hepatocellular carcinoma (TACTICS-L) in Japan: Final analysis. J Clin Oncol 2022;40 Suppl:Abstract no. 417.

53. Shimose S, Sugimoto R, Hiraoka A, Tanaka M, Iwamoto H, Tanaka Y, et al. Significance of ramucirumab following atezolizumab plus bevacizumab therapy for hepatocellular carcinoma using real-world data. Hepatol Res 2023;53:116-126.



54. Kuzuya T, Kawabe N, Hashimoto S, Funasaka K, Nagasaka M, Nakagawa Y, et al. Clinical outcomes of ramucirumab as posttreatment following atezolizumab/bevacizumab combination therapy in advanced hepatocellular carcinoma. Anticancer Res 2022;42:1905-1910.


55. Komatsu S, Yano Y, Fujishima Y, Ishida J, Kido M, Kuramitsu K, et al. Current role of atezolizumab plus bevacizumab therapy in the sequential treatment of unresectable hepatocellular carcinoma. Anticancer Res 2022;42:1403-1412.


56. Kato N, Kudo M, Tsuchiya K, Hagihara A, Numata K, Aikata H, et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: Final results of a multicenter phase II study. Hepatol Res 2023 Jan 5;doi: 10.1111/hepr.13876.



57. Calderaro J, Seraphin TP, Luedde T, Simon TG. Artificial intelligence for the prevention and clinical management of hepatocellular carcinoma. J Hepatol 2022;76:1348-1361.


58. Murai H, Kodama T, Maesaka K, Tange S, Motooka D, Suzuki Y, et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology 2023;77:77-91.




59. Sato M, Tateishi R, Yatomi Y, Koike K. Artificial intelligence in the diagnosis and management of hepatocellular carcinoma. J Gastroenterol Hepatol 2021;36:551-560.



60. Nishida N, Yamakawa M, Shiina T, Mekada Y, Nishida M, Sakamoto N, et al.; JSUM A. I. investigators. Artificial intelligence (AI) models for the ultrasonographic diagnosis of liver tumors and comparison of diagnostic accuracies between AI and human experts. J Gastroenterol 2022;57:309-321.




61. Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, et al. Off-the-shelf V╬┤1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer 2021;9:e003441.

-
METRICS

- ORCID iDs
-
Hironori Koga

https://orcid.org/0000-0001-5814-9543 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



