| Clin Mol Hepatol > Volume 22(2); 2016 > Article |
|
ABSTRACT
Laparoscopic liver resection (LLR) is becoming widely accepted for the treatment of hepatocellular carcinoma (HCC). Laparoscopic left lateral sectionectomy and minor laparoscopic liver resection are now considered standard approaches, especially for tumors located in the anterolateral segments of the liver. Laparoscopic left lateral sectionectomy in adult donors is also gaining acceptance for child liver transplantation in many centers. Major LLRs, including left hepatectomy and right hepatectomy, have been recently attempted. Laparoscopic donor hepatectomy is becoming more popular owing to increasing demand from young living donors who appreciate its minimal invasiveness and excellent cosmetic outcomes. Several centers have performed total laparoscopic donor right hepatectomy in adult-to-adult living donor liver transplantation. Many meta-analyses have shown that LLR is better than open liver resection in terms of short-term outcomes, principally cosmetic outcomes. Although no randomized control trials have compared LLR with open liver resection, the long-term oncologic outcomes were similar for both procedures in recent case-matched studies.
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor worldwide, accounting for 5.6% of all human cancers, and is the most common primary liver cancer [1]. It is also the third most common cause of cancer-related deaths worldwide [2]. The number of new cases is estimated to range from 500,000 to 1 million per year [1]. Up to 80ŌĆō90% of HCCs develop in a cirrhotic liver [2].
Liver transplantation appears to be effective treatment approach because it treats both the cancer and the underlying liver cirrhosis. However, the scarcity of donors precludes transplantation in all patients with early HCC [2]. Liver resection for HCC is now considered to be a safer procedure than was previously believed owing to technical advances and improvements in postoperative patient management [3-6]. Accordingly, in many centers, liver resection is still the first-line treatment for HCC in patients with compensated cirrhosis [7]. Since the first laparoscopic liver wedge resection was reported in 1992, an increasing number of reports have described the feasibility, safety, and adequacy of laparoscopic hepatic procedures [8-11]. Now, laparoscopic liver resection (LLR) is commonly performed in patients with HCC and chronic liver disease.
The indications for LLR have been changed substantially since its introduction. Initially, LLR was limited to the treatment of benign diseases. However, with increasing know-ledge of this procedure, its indications have widened to include malignant disease such as HCC and liver metastasis of colorectal cancer. The extent of resection has also grown over time [12]. Major liver resection, such as right or left hemihepatectomy, has been performed more frequently in recent years [13,14].
Laparoscopic left lateral sectionectomy is now regarded as a standard treatment option. By contrast, it will take many years for LLR to become a standard procedure for treating all kinds of HCC [15]. Extending the indications, the introduction of advanced techniques, and outcomes similar to those of open liver resection (OLR) are required for LLR to become a standard procedure in HCC [9].
The aim of this review is to assess the current indications, advantages, and limitations of laparoscopic surgery for HCC resection. We will also discuss the feasibility of LLR and its oncologic outcomes compared to OLR. The information in this review was extracted from a literature search of Medline.
Unlike laparoscopic cholecystectomy, laparoscopy is not widely accepted for liver resection because of the technical difficulty associated with parenchymal transection, hemostasis at the transection plane, the risk of air embolism, and limited ability to explore the deeper regions of the liver [16]. Therefore, LLR has been reserved for patients who require limited resection of tumors located on the left side of the liver. The recent improvements in laparoscopic techniques and the introduction of new technologies mean that LLR is technically feasible and safe for tumors on the right side of the liver [17]. The first international position statement on LLR published in 2008 stated that the best indications for LLR were patients with solitary lesions, Ōēż 5 cm in diameter, located in the peripheral liver segments (i.e. segments 2ŌĆō6; Fig. 1). Laparoscopic left lateral sectionectomy should be considered as the standard of care, but major hepatectomy, such as right hepatectomy, should be reserved for experienced surgeons [15].
Improved laparoscopic techniques, better visualization of the operative field using a flexible laparoscope, and routine use of a laparoscopic cavitron ultrasonic surgical aspirator for transecting the deeper portion of the liver parenchyma have allowed laparoscopic left lateral sectionectomy to be performed more widely [18-20]. LLR for HCC located in the posterosuperior segments in selected patients was reported to be safe and feasible, and offered comparable oncologic outcomes to those of OLR. Other benefits of LLR include reduced blood loss, fewer complications, and shorter postoperative hospital stay compared with open resection [21].
Cirrhosis precedes HCC in approximately 80%ŌĆō90% of cases worldwide [22]. Asian countries, especially, have a disproportionately high prevalence of HCC, mainly because hepatitis B and C viruses are endemic in these countries [23], and chronic infection is associated with high risk of liver cirrhosis and HCC [24]. When considering liver resection in patients with liver cirrhosis, it is important to consider the degree of surgical stress placed on the patient and the liver, as well as the oncological outcomes [8]. Decompensated cirrhosis is generally considered to be a contraindication to liver resection and thereby LLR [25]. Uncontrolled portal hypertension, including esophageal varices and low platelet count, is also usually considered as an exclusion criterion for LLR [26]. Anatomical liver resection is preferred for HCC because of its tendency to invade the portal veins and spread along the intrasegmental branches [27].
Major advantages of laparoscopy are the rapid recovery of patients and the shorter hospital stay compared with open surgery, as previously reported for LLR of HCC [28,29]. These advantages are related to less postoperative pain, early ambulation, early return of oral feeding, and lower incidence of postoperative complications after LLR. Another important advantage of LLR in cirrhotic patients is the lower incidence of postoperative liver failure and ascites. This may be due to the reduced invasiveness of laparoscopy, which helps to preserve the abdominal musculature by avoiding large abdominal incisions, preserve the parietal circulation, and minimize liver manipulation [8].
Because the potential applications for LLR have expanded considerably in the last 15 years [28,30,31], an first International Consensus Conference on LLR was convened in Louisville, Kentucky, in 2008 [15]. The experts discussed achievements and recommendations for this approach [15]. This consensus statement defined the current international position on laparoscopic liver surgery as ŌĆ£a safe and effective approach for the management of surgical liver disease in the hands of trained surgeons with experience in hepatobiliary and laparoscopic surgery.ŌĆØ It also stated that the best indications for LLR were patients with solitary lesions, Ōēż 5 cm in diameter, located in the peripheral liver segments (i.e. segments 2ŌĆō6) and that laparoscopic left lateral sectionectomy should be considered as the standard of care. If local resection of HCC is performed, it should involve anatomical segmental resection, if possible, considering the overall function of the liver. This is because this procedure is associated with lower local recurrence rates and should be used instead of tumorectomy. Since then, LLR has been introduced to middle-tier centers as well as high-volume and/or specialized centers [32]. Moreover, the number of HCC cases treated by LLR has increased over the last 5 years, especially in Asia and Europe [33].
Six years later, the second International Consensus Conference on LLR was held to evaluate the current status of LLR and to develop recommendations and guidelines. This goal was achieved through analysis of the available literature and expert presentations, which including videos presented to an independent jury. The organizing committee invited 43 respected surgeons from 18 countries. The expert panel comprised 34 members, with demonstrated experience in LLR, and the jury contained 9 members. The expert panel provided evidence and developed recommendations. The organizing committee prepared 17 questions in 2 categoriesŌĆöbenefits and risks, and techniques of LLR. Each question was assigned to a working group of 3ŌĆō7 members of the expert panel who were selected based on their scientific and clinical activities. The jury concluded that minor LLRs had become standard practice (IDEAL 3) and that major liver resections were innovative procedures in the exploratory phase (IDEAL 2b). Continued cautious introduction of major LLRs was recommended. All of the evidence available for scrutiny was considered to be of low quality by GRADE, which prompted the recommendation for higher quality evaluative studies. The expert panel developed recommendations regarding preoperative evaluation, bleeding control, transection methods, anatomical approaches, and equipment. Both the expert panel and jury recognized the need for a formal structure of education for surgeons interested in performing major LLR because of the steep learning curve [34].
Over the past decade, LLR has progressed internationally following advances in technology and the increasing experience of liver surgeons. Indeed, more than 9,000 procedures were reported in the English literature [35]. With the proper selection of patients, LLR is considered as a safe technique, with mortality and morbidity rates of 0% and 15%, respectively [36]. Since the first case was reported, an increasing number of case-series have been published especially from the beginning of new millenium [37]. LLR was initially performed for low-risk operations, including the excision of benign hepatic lesions. The techniques have gradually become incorporated into the practices of most liver centers, and LLR is now widely accepted for the management of benign and malignant liver tumors [38]. In a global survey of the current practices of liver surgery, Yoshihiro et al. reported that 88% of the participating centers had incorporated laparoscopic approaches into liver surgery [33].
To our knowledge, no randomized controlled trials (RCT) have compared the outcomes between LLR and OLR. However, several retrospective caseŌĆōcohort matched studies have compared these two procedures. The majority of studies showed that LLR has major benefits compared with OLR. LLR was associated with less intraoperative blood loss, less postoperative pain medication requirement, earlier return of oral feeding, and shorter hospital stay compared with OLR. In addition, from a financial standpoint, although the minimally invasive LLR approach was associated with higher operating room costs in some studies, the total hospital costs were either offset or improved by LLR because of the shorter hospital stay. In addition, LLR did not compromise oncological measures such as margin status, disease-free survival, or overall survival, but did improve short-term perioperative outcomes [39].
A systematic review published in 2012 compared LLR with OLR [41]. The data analysis suggested that LLR was associated with improvements in most of the perioperative factors, including blood loss, the number of patients requiring transfusion, and the use of portal triad clamping. By contrast, the operation time was shorter with OLR than with LLR. LLR was also associated with shorter hospital stay and earlier return of oral feeding. However, all of these significant results were associated with significant heterogeneity in the evaluated studies. There were no differences between the two groups in terms of adverse outcomes in the early postoperative period. Nevertheless, a significant finding was the lower number of positive resection margins in the LLR group than in the OLR group. This finding was not associated with significant heterogeneity. The other variables associated with oncological clearance were not significantly different between LLR and OLR. Another important result was that LLR was associated with a significant reduction in overall morbidity compared with OLR.
In the last 5 years, several meta-analyses of studies comparing LLR and OLR for malignant lesions have been published (Table 1). All of these meta-analyses concluded that LLR is superior to OLR in terms of perioperative outcomes. The operation time was not significantly different between LLR and OLR, even though operation time was shorter for OLR in prior studies. The absence of a difference in the meta-analyses could be explained by recent advances in surgical instruments, accumulated experience, and overcoming the learning curve. Furthermore, no technique compromised the oncological outcomes [40-45]. Unfortunately, it is impossible to reach a convincing conclusion regarding the bene-fits and risks of LLR over OLR in the absence of RCTs [46]. However, Abraham et al. recently reported that a meta-analysis of well-designed non-randomized controlled trials of surgical procedures is probably as reliable as a meta-analysis of RCTs [47].
LLR not only achieve equivalent short-term postoperative outcomes but also provide favorable long-term survival prognosis to OLR. Several studies have reported that LLR is less invasive and is associated with similar disease-free survival and overall survival rates to OLR in patients with HCC [48,49]. However, we still lack data on the long-term oncological outcomes of LLR particularly in patients with HCC. To date, there have been no prospective RCT comparing the outcomes between LLR and OLR. However, many meta-analyses, retrospective studies, and case-matched studies with propensity score matching comparing the long-term outcomes of LLR and OLR in patients with HCC have been published in recent years (Table 2). These studies showed that the survival rates at 1, 3, and 5 years were similar between patients undergoing LLR and patients undergoing OLR for HCC, as were the overall recurrence rate, mortality rate, overall survival rate, and disease-free survival time [46,50-54]. One study determined the long-term survival of patients with HCC in reference to the stage of the disease. Survival was not significantly different between patients with stage I and stage II HCC.
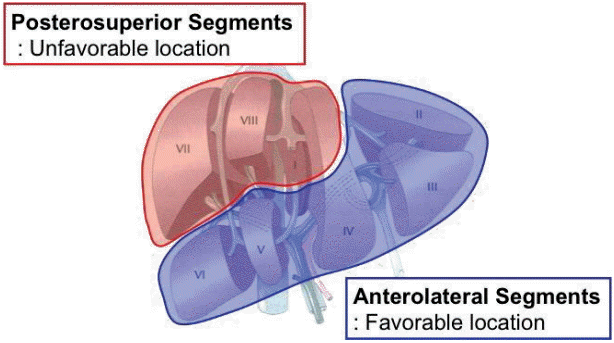
Figure┬Ā1.
The peripheral area of anterolateral segments (segments 2, 5, 6, and lower part of 4) is considered to be a favorable location of tumors for laparoscopic liver resection, whereas the posterosuperior segments (segments 1, 7, 8, and upper part of 4) of the liver are unfavorable locations.
Table┬Ā1.
Previous studies comparing the outcomes of laparoscopic liver resection versus open resection.
| Author | Type | Blood loss | Transfusion | Operative time | Hospital stay | Complications | Resection margin |
|---|---|---|---|---|---|---|---|
| Zhou et al. [40] (2011) | Meta-analysis 21 studies | LLR < OLR | LLR < OLR | NSD | LLR < OLR | LLR < OLR | NSD |
| Rao et al. [41] (2011) | Systematic review 10 studies | LLR < OLR | LLR < OLR | NSD | LLR < OLR | LLR < OLR | NSD |
| Fancellu et al. [42] (2011) | Meta-analysis 9 studies | LLR < OLR | LLR < OLR | NSD | LLR < OLR | LLR < OLR | NSD |
| Li et al. [43] (2012) | Meta-analysis 10 studies | LLR < OLR | LLR < OLR | NSD | LLR < OLR | LLR < OLR | NSD |
| Xiong et al. [44] (2012) | Meta-analysis 16 studies | LLR < OLR | LLR < OLR | NSD | LLR < OLR | LLR < OLR | NSD |
| Yin et al. [45] (2013) | Meta-analysis 15 studies | LLR < OLR | LLR < OLR | NSD | LLR < OLR | LLR < OLR | NSD |
Table┬Ā2.
Recent studies on long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma
| Study | Type | 1 year survival | 3 year survival | 5 year survival | 1 year DFS | 3 year DFS | 5 year DFS | Overall and DFS |
|---|---|---|---|---|---|---|---|---|
| Lee et al. [50] (2011) | Case matched | L - 86.9% | L ŌĆō 81.8% | L ŌĆō 76% | L ŌĆō 78.8% | L ŌĆō 51% | L ŌĆō 45.3% | NSD |
| O - 98% | O ŌĆō 80.6% | O ŌĆō 76.1% | O ŌĆō 69.2% | O ŌĆō 55.9% | O ŌĆō 55.9% | |||
| Parks et al. [51] (2014) | Meta-analysis | L ŌĆō 92% | L ŌĆō 77.7% | L ŌĆō 61.9% | NA | NA | NA | NA |
| O ŌĆō 91.3% | O ŌĆō 76.5% | O ŌĆō 56.5% | ||||||
| Cheung et al. [52] (2013) | Retrospective | L ŌĆō 96.6% | L ŌĆō 87.5% | L ŌĆō 76.6% | L ŌĆō 87.3% | L ŌĆō 72.6% | L ŌĆō 54.5% | NA |
| O ŌĆō 95.2% | O ŌĆō 72.9% | O ŌĆō 57% | O ŌĆō 63.5% | O ŌĆō 50% | O ŌĆō 44.3% | |||
| Kim et al. [53] (2014) | Case matched with PSM | L ŌĆō 100% | L ŌĆō 100% | L ŌĆō 92.2% | L ŌĆō 81.7% | L ŌĆō 61.7% | L ŌĆō 54% | NSD |
| O ŌĆō 96.5% | O ŌĆō 92.2% | O ŌĆō 87.7% | O ŌĆō 78.6% | O ŌĆō 60.9% | O ŌĆō 40.1% | |||
| Han et al. [54] (2015) | Case matched with PSM | L ŌĆō 91.6% | L ŌĆō 87.5% | L ŌĆō 76.4% | L ŌĆō 69.7% | L ŌĆō 52% | L ŌĆō 44.2% | NSD |
| O ŌĆō 93.1% | O ŌĆō 87.8% | O ŌĆō 73.2% | O ŌĆō 74.7% | O ŌĆō 49.5% | O ŌĆō 41.2% | |||
| Takahara et al. [46] (2015) | Case matched with PSM | L ŌĆō 95.8% | L ŌĆō 86.2% | L ŌĆō 76.8% | L ŌĆō 83.7% | L ŌĆō 58.3% | L ŌĆō 40.7% | NSD |
| O ŌĆō 95.8% | O ŌĆō 84% | O ŌĆō 70.9% | O ŌĆō 79.6% | O ŌĆō 50.4% | O ŌĆō 39.3% |
REFERENCES
1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-2576.


2. Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48(Suppl 1):S20-S37.


3. Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-330.



4. Jaskille A, Schechner A, Park K, Williams M, Wang D, Sava J. Abdominal insufflation decreases blood loss and mortality after porcine liver injury. J Trauma 2005;59:1305-1308 discussion 1308.


5. Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 2001;234:63-70.



6. Belghiti J, Regimbeau JM, Durand F, Kianmanesh AR, Dondero F, Terris B, et al. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology 2002;49:41-46.

7. Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol 2014;20:4892-4899.



8. Shehta A, Han HS, Yoon YS, Cho JY, Choi Y. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc 2016;30:638-648.



9. Han HS, Yoon YS, Cho JY, Hwang DW. Laparoscopic liver resection for hepatocellular carcinoma: korean experiences. Liver Cancer 2013;2:25-30.



10. Tzanis D, Shivathirthan N, Laurent A, Abu Hilal M, Soubrane O, Kazaryan AM, et al. European experience of laparoscopic major hepatectomy. J Hepatobiliary Pancreat Sci 2013;20:120-124.


11. Ettorre GM, Levi Sandri GB, Santoro R, Vennarecci G, Lepiane P, Colasanti M, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: single center experience of 90 cases. Hepatobiliary Surg Nutr 2015;4:320-324.


12. Koffron A, Geller D, Gamblin TC, Abecassis M. Laparoscopic liver surgery: Shifting the management of liver tumors. Hepatology 2006;44:1694-1700.


13. Park JS, Han HS, Hwang DW, Yoon YS, Cho JY, Koh YS, et al. Current status of laparoscopic liver resection in Korea. J Korean Med Sci 2012;27:767-771.



14. Hwang DW, Han HS, Yoon YS, Cho JY, Kwon Y, Kim JH, et al. Laparoscopic major liver resection in Korea: a multicenter study. J Hepatobiliary Pancreat Sci 2013;20:125-130.


15. Buell JF, Cherqui D, Geller DA, OŌĆÖRourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-830.


16. Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery 2004;136:804-811.


17. Cho JY, Han HS, Yoon YS, Shin SH. Outcomes of laparoscopic liver resection for lesions located in the right side of the liver. Arch Surg 2009;144:25-29.


18. Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 2008;144:32-38.


19. Cho JY, Han HS, Yoon YS, Shin SH. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc 2008;22:2344-2349.


20. Yoon YS, Han HS, Cho JY, Ahn KS. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc 2010;24:1630-1637.


21. Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc 2015;29:2994-3001.


22. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127(5 Suppl 1):S35-S50.


23. Kao JH. Risk stratification of HBV infection in Asia-Pacific region. Clin Mol Hepatol 2014;20:223-227.



24. Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009;10:1111-1118.


25. Soubrane O, Goumard C, Laurent A, Tranchart H, Truant S, Gayet B, et al. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB (Oxford) 2014;16:357-365.



26. Dagher I, Belli G, Fantini C, Laurent A, Tayar C, Lainas P, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 2010;211:16-23.


27. Makuuchi M, Imamura H, Sugawara Y, Takayama T. Progress in surgical treatment of hepatocellular carcinoma. Oncology 2002;62(Suppl 1):74-81.


28. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-841.


29. Belli G, Limongelli P, Fantini C, DŌĆÖAgostino A, Cioffi L, Belli A, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 2009;96:1041-1048.


30. Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 2008;248:475-486.


31. Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007;246:385-392 discussion 392-384.



32. Hibi T, Cherqui D, Geller DA, Itano O, Kitagawa Y, Wakabayashi G. International Survey on Technical Aspects of Laparoscopic Liver Resection: a web-based study on the global diffusion of laparoscopic liver surgery prior to the 2nd International Consensus Conference on Laparoscopic Liver Resection in Iwate, Japan. J Hepatobiliary Pancreat Sci 2014;21:737-744.


33. Mise Y, Sakamoto Y, Ishizawa T, Kaneko J, Aoki T, Hasegawa K, et al. A worldwide survey of the current daily practice in liver surgery. Liver Cancer 2013;2:55-66.



34. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-629.

35. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-777.


36. Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg 2009;250:103-111.


37. Piardi T, Sommacale D, Baumert T, Mutter D, Marescaux J, Pessaux P. Laparoscopic resection for hepatocellular carcinoma: comparison between Middle Eastern and Western experience. Hepatobiliary Surg Nutr 2014;3:60-72.


38. Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc 2008;22:2208-2213.


39. Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-356.


40. Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011;56:1937-1943.


41. Rao A, Rao G, Ahmed I. Laparoscopic or open liver resection? Let systematic review decide it. Am J Surg 2012;204:222-231.


42. Fancellu A, Rosman AS, Sanna V, Nigri GR, Zorcolo L, Pisano M, et al. Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J Surg Res 2011;171:e33-45.


43. Li N, Wu YR, Wu B, Lu MQ. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: A meta-analysis. Hepatol Res 2012;42:51-59.


44. Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 2012;18:6657-6668.



45. Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 2013;20:1203-1215.


46. Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721-727.


47. Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 2010;63:238-245.


48. Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 2014;20:8274-8281.



49. Ahn KS, Kang KJ, Kim YH, Kim TS, Lim TJ. A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A 2014;24:872-877.


50. Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg 2011;35:2268-2274.


51. Parks KR1, Kuo YH, Davis JM, OŌĆÖ Brien B, Hagopian EJ. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB (Oxford) 2014;16:109-118.



52. Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 2013;257:506-511.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




