Autophagy and liver cancer
Article information
Abstract
Autophagy is a highly conserved catabolic process that degrades cytosolic proteins and organelles via formation of autophagosomes that fuse with lysosomes to form autolysosomes, whereby autophagic cargos are degraded. Numerous studies have demonstrated that autophagy plays a critical role in the regulation of liver physiology and homeostasis, and impaired autophagy leads to the pathogenesis of various liver diseases such as viral hepatitis, alcohol associated liver diseases (AALD), non-alcoholic fatty liver diseases (NAFLD), and liver cancer. Recent evidence indicates that autophagy may play a dual role in liver cancer: inhibiting early tumor initiation while promoting progression and malignancy of already formed liver tumors. In this review, we summarized the progress of current understanding of how hepatic viral infection, alcohol consumption and diet-induced fatty liver diseases impair hepatic autophagy. We also discussed how impaired autophagy promotes liver tumorigenesis, and paradoxically how autophagy is required to promote the malignancy and progression of liver cancer. Understanding the molecular mechanisms underlying how autophagy differentially affects liver cancer development and progression may help to design better therapeutic strategies for prevention and treatment of liver cancer.
INTRODUCTION
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved catabolic process for maintaining cellular homeostasis under both basal and stress conditions. Autophagy facilitates the clearance or turnover of long-lived or misfolded proteins, insoluble protein aggregates, invading microbes and damaged or excess organelles [1-3]. Autophagy has generally been thought to play dual roles in tumorigenesis and cancer progression. Autophagy serves as a tumor suppressor to inhibit tumorigenesis by removing damaged organelles and reducing oxidative stress to mitigate DNA damage and genome instability. However, after the cells have transformed and become cancerous, they can use autophagy as a survival mechanism against the harsh environment characterized by a lack of both oxygen and nutrients. As a result, blocking autophagy in combination with other chemotherapeutic drugs is believed to be a promising therapeutic strategy to treat various cancers [4].
Liver cancer is the third leading cause of cancer-related deaths worldwide and accounts for more than 700,000 deaths annually [5,6]. According to a recent study, the mortality rate of hepatocellular carcinoma (HCC) in the USA increased approximately 40% between 2000 and 2016 [7]. As a multistage disease, HCC development is linked to many environmental risk factors such as alcohol associated liver disease (AALD), hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, aflatoxin B1 and obesity-related nonalcoholic fatty liver diseases (NAFLD) [8]. Interestingly, among the high-risk factors of HCC, impaired hepatic autophagy has been shown to contribute to the pathogenesis of AALD, NAFLD as well as HBV and HCV hepatitis [9-14], suggesting that defective autophagy may be a common mechanism for HCC development. In this review, we briefly discuss the current understanding of the role and mechanisms of autophagy in the pathogenesis of liver cancer.
DUAL ROLES OF AUTOPHAGY IN LIVER TUMORIGENESIS AND CANCER PROGRESSION
As mentioned above, autophagy may play dual roles in cancer depending on the stage of tumor development. Autophagy serves as a tumor suppressor to inhibit tumorigenesis likely before the normal cells are transformed into cancer cells. Autophagy also promotes cancer progression and malignancy in already transformed cancer cells. Below we will discuss in detail the paradoxical role of autophagy in tumor initiation and progression. Understanding the complex dual roles of autophagy in cancer has very important implications as it will help to redefine the context in which autophagy manipulation can act as tumor prevention or treatment/therapy. For instance, agents that increase autophagy activity may be used to prevent tumorigenesis whereas agents that inhibit autophagy may be used to treat existing cancers.
AUTOPHAGY INHIBITS LIVER TUMORIGENESIS
The first direct evidence to support autophagy as a tumor suppressor came from the pioneering work of Dr. Beth Levine’s group, who observed spontaneous tumor occurrence in multiple tissues, including the liver, in aged beclin1 heterozygous mice [15,16]. This result was later confirmed by Yue et al. [17]. The beclin1 heterozygous mice are also highly susceptible to HCC upon HBV infection [16]. Moreover, beclin1 is frequently found mono-allelically deleted in many human cancers such as prostate, ovarian and breast cancers [15] suggesting that Beclin1 inhibits tumorigenesis and acts as a haploinsufficient tumor suppressor. Further studies show that decreased expression of beclin1 is correlated with HCC grade, suggesting its possibility as a HCC prognostic bio-marker [18]. However, beclin1 has a BH3 domain and interacts with Bcl-2 protein, a critical regulator of cell death. Moreover, beclin1 homozygous knockout (KO) mice are embryonic lethal. This is in great contrast to the phenotypes of homozygous deletion of other Atgs such as atg5 or atg7 in mice, which die at neonatal stage with normal embryo development [19,20]. Therefore, it has been questioned whether the tumor suppressive function of Beclin1 was directly due to its inhibition of autophagy or its non-autophagic functions. Subsequent studies from Komatsu’s and Mizushima’s group as well as ours show that liver-specific atg5 or atg7 KO mice also develop spontaneous liver tumors [21-23], confirming that autophagy is indeed a bona fide tumor suppressor. The tumor suppressive function of autophagy is likely due to its ability to remove cellular protein aggregates and damaged organelles. The failure to remove damaged organelles such as mitochondria and protein aggregates in a timely manner can lead to a cascade of increased oxidative and metabolic stress, genome instability, and ultimately, cell malignant transformation [24-28]. Indeed, liver-specific atg5 or atg7 KO mice have increased hepatic oxidative DNA damage, accumulation of protein aggregates and damaged mitochondria as well as an increased number of peroxisomes and endoplasmic reticulum (ER) content, increased apoptosis, compensatory hepatocyte proliferation, hepatomegaly, increased ductular reaction, inflammation, and fibrosis, all of which can result in the development of liver tumors [21,23,29-32]. Several signaling pathways have been identified that may contribute to the liver pathogenesis and tumorigenesis in autophagy-defective liver including high mobility group box 1 (HMGB1), yes-associated protein (Yap), mechanistic target of rapamycin (mTOR), and p62-nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway [32-35]. Among these signaling pathways in autophagy-defective liver, the p62-Nrf2 pathway seems to be the central player and has a more profound impact on liver pathogenesis and tumorigenesis than HMGB1, Yap, or mTOR, is discussed in detail later.
HMGB1 is a non-histone DNA binding protein and can be released into the extracellular space either passively during cell death or actively following cytokine stimulation. Once released, HMGB1 acts as a damage-associated molecular pattern (DAMP) molecule to activate immune cells [36,37], which is associated with liver injury, sepsis, inflammation, and fibrosis [38,39]. Deletion of hmgb1 in liver-specific atg7 KO mice markedly attenuated ductular reaction and liver tumorigenesis but did not improve liver injury, hepatomegaly, inflammation or fibrosis in these mice [33].
Yap is a key component of the Hippo signaling pathway and acts as a transcriptional co-activator. Yap binds to the transcriptional enhanced associate domain (TEAD) family of transcription factors and regulates the expression of a set of genes for cell proliferation, anti-apoptosis and “stemness [40,41]”. Lee et al. [34] found that liver-specific atg7 KO mice have increased hepatic cytoplasmic and nuclear Yap protein accumulation and increased expression of Yap target genes. They further found that Yap colocalizes with GFP-LC3 positive autophagosomes and lysotracker positive lysosomes in hepatocytes. Moreover, Yap protein increases when autophagy is inhibited either pharmacologically or genetically, suggesting that Yap protein may be a novel autophagy substrate. Deletion of yap in liver-specific-atg7 KO mice leads to a decrease in hepatocyte size, hepatomegaly, inflammation, ductular reaction, progenitor cell expansion and fibrosis. More importantly, the number and size of liver tumors decreases but are not eliminated in yap/atg7 double KO mice [34].
mTOR is a key cellular nutrient and energy sensor that regulates the cellular anabolic pathway by increasing protein and lipid synthesis to meet the demand for cell proliferation. Mammalian cells have two mTOR complexes: mTOR complex1 (mTORC1) and mTORC2, in which mTORC1 is rapamycin-sensitive but mTORC2 is relatively rapamycin resistant. Both mTORC1 and mTORC2 share several common components such as the mTOR kinase but also have distinct subunits. For instance, mTORC1 has the scaffold protein Raptor whereas mTORC2 contains Rictor [42]. As cancer cells have a higher proliferation rate, it is not surprising that approximately 50% human cancers, including HCC, have aberrant mTOR activation [43]. Increased mTOR activation in HCC is mainly attributed to loss of function mutations of phosphatase and tensin homolog (PTEN), tuberous sclerosis complex 1 (TSC1) or TSC2 [44,45]. Mice with liver-specific deletion of pten or tsc1 have persistent hepatic mTOR activation, hepatomegaly, and develop spontaneous tumors [46]. Therefore, targeting mTOR has been an attractive approach for treating various cancers. However, results from clinical trials using rapamycin and its pharmacological analogs are not promising as only minimal beneficial effects are observed [47,48]. We recently demonstrated that genetic ablation of mtor has a dual role in the liver pathogenesis and tumorigenesis of L-atg5 KO mice. Deletion of mtor decreases hepatomegaly, cell death and inflammation but not fibrosis in young (2 months-old) L-atg5 KO mice. However, these beneficial effects are gradually lost in older (6–12 months-old) L-atg5/mtor DKO mice as they all develop tumors at 12 months-old. Moreover, more than 50% of L-atg5/mtor DKO mice develop spontaneous tumors but none of the L-atg5 KO mice have tumors at 6 months-old. These results suggest that ablation of mtor exacerbates tumorigenesis in autophagy defective mouse livers. Mechanistically, we found that L-atg5/mtor DKO mice have increased protein kinase B (PKB, also known as AKT) activation compared with L-atg5 KO, suggesting that the early development of liver tumors could be mediated by compensatory AKT activation in the absence of both mTOR and autophagy. It is possible that pharmacological inhibiting both mTOR and AKT may have increased beneficial effects compared to targeting mTOR alone for treating HCC.
Taken together, it seems that HMGB1, Yap, and mTOR all contribute to tumorigenesis in autophagy defective livers although ablation of individual proteins cannot completely eliminate liver tumors, which is in contrast with the ablation of Nrf2 that completely abolishes liver tumors in autophagy defective livers (see below discussion).
AUTOPHAGY PROMOTES MALIGNANT TUMOR PROGRESSION
Perhaps one of the most intriguing findings from the liver-specific atg5 or atg7 KO mice is that these hepatic autophagy defective mice develop benign adenomas but not malignant HCC [21,23]. Notably, liver-specific atg5 KO mice fail to develop HCC even after they were exposed to the hepatic carcinogen diethylnitrosamine (DEN) [29]. These data may suggest that during the late tumor progression stage, autophagy may be required for benign tumors to progress to malignant tumors. One possible mechanism is that loss of hepatic autophagy leads to the induction of several tumor suppressor genes, such as p53, p21, p27, p16, and PTEN [29,32]. Increased p53 in autophagy deficient hepatoma cells negatively regulates Nanog homeobox (NANOG) and cancer stem cell (CSC) proliferation, which may suppress tumor progression [49]. Mechanistically, it is known that mitophagy, a selective autophagy for mitochondrial removal, positively regulates hepatic CSC by suppressing the tumor suppressor p53. When mitophagy is induced, p53 colocalizes with mitochondria and is removed in a mitophagy-dependent manner. However, when mitophagy or autophagy is inhibited, mitochondrial p53 is phosphorylated by PTEN-induced kinase 1 (PINK1) and translocated into the nucleus. Once in the nucleus, p53 binds to the NANOG promoter to prevent OCT4 and SOX2 transcription factors from activating the expression of NANOG, a transcription factor critical for maintaining the stemness and self-renewal ability of CSCs, resulting in the reduction of hepatic CSC populations. These results demonstrate that autophagy, and more likely mitophagy, controls the activities of p53 to maintain hepatic CSC and provides an explanation as to why autophagy is required to promote hepatocarcinogenesis [49,50]. However, it remains to be studied whether further deletion of tumor suppressor genes such as Trp53 or pten in liver-specific atg5 or atg7 KO mice would promote the progression of benign tumors to malignant tumors.
Increasing evidence has demonstrated that autophagy is robustly activated in tumor cells including HCC under a multitude of stressors, such as starvation, growth factor deprivation, hypoxia, proteasome inhibition and therapeutic agents [51]. Inducible autophagy constitutes an important pro-survival mechanism for cancer cells by removing toxic oxygen radicals or damaged misfolded proteins, relieving ER stress, maintaining mitochondrial function, sustaining metabolism, and preventing diversion of tumor progression to benign oncocytomas [51,52-56]. It is reported that autophagosome formation is induced in hypoxic tumor regions and that Beclin1 deletion leads to tumor cell death specifically in these hypoxic regions [57,58]. Moreover, inhibition of autophagy restores the sensitivity of hepatoma cells to chemotherapy, suggesting that autophagy plays a pro-survival role in chemotherapeutic agent-induced cell death [57]. In many other cancer cells, such as pancreatic cancer, elevated basal autophagy is required for continued cell growth and maintaining the intracellular amino acid pool, suggesting that tumor cells have evolved to rely on autophagy even under basal conditions [59,60]. In KrasG12D- or BrafV600E-driven lung cancer, KrasG12D-driven pancreatic ductal adenocarcinoma (PDAC), or pten–/–-driven prostate cancer, deletion of atg5 or atg7 decreases tumor progression [61-64], further supporting the notion that functional autophagy is required for tumor progression.
While cancer cell autophagy is important for cancer cell survival, emerging evidence now suggests that host autophagy also plays a critical role in tumor growth. It should be noted that tumors obtain their nutrient supply from the host and thus whole-body metabolism and the tumor microenvironment are critical for tumor growth. To address host autophagy in metabolism and its impact on tumor growth, Dr. Eileen White’s group established an animal model that allograft autophagy-competent cancer cell lines onto autophagy wild-type and autophagy-deficient (inducible wholebody atg7 deleted) host mice [65]. Loss of host autophagy markedly impairs growth of multiple different allografted tumors due to decreased levels of circulating arginine. Arginine is a non-essential amino acid, which has to be obtained from the diet, de novo synthesis or protein turnover. Arginine is also important for mTOR activation to promote tumor cell growth. Interestingly, some human cancer cells have silenced the expression of the enzyme argininosuccinate synthase (ASS1) for arginine synthesis that render these cancer cells sensitive to arginine deficiency [66]. Circulating arginine is generally broken down by arginase I (ARG1), an enzyme predominantly expressed in liver and released from hepatocytes into circulation. Host-atg7 KO mice have increased liver injury (similar to L-atg7 KO mice) resulting in increased release of ARG1 into circulation. Dietary supplementation of arginine for host-atg7 KO mice partially restored circulating arginine levels and tumor growth. Ablation of host autophagy by conditional induction of systemic expression of a dominant-negative ATG4b in mice with KrasG12D- and Trp53–/+-driven PDAC also leads to inhibition of PDAC progression [67]. These results indicate that systemic inhibition of autophagy may be beneficial for cancer treatment at multiple levels by targeting both cancer cells and host cells.
The tumor microenvironment is an integral part of the tumor, which is composed of cancerous and noncancerous cells (e.g., immune cells, stromal cells, endothelial cells, and adipocytes) as well as various mediators (e.g., cytokines, chemokines, growth factors, and humoral factors). The tumor microenvironment plays a critical role in cancer cell adaptation, growth and progression as well as resistance to therapies. Autophagy in the tumor microenvironment can provide the cancer cells/tumor cells with amino acids, extracellular matrix molecules and cytokines (e.g., interleukin-6) to promote tumor growth [68,69]. In addition, autophagy may also affect the anti-tumor T cell responses and reduce tumor growth in syngeneic host mice. In PDAC, autophagy selectively degrades major histocompatibility complex class I (MHC-I) via autophagy receptor protein NBR1, which results in decreased surface levels of MHC-1 on PDAC cells and reduced CD8+ T cells-mediated antitumor immunity. Inhibition of autophagy in PDAC cells leads to increased antigen presentation, enhanced CD8+ T cell proliferation and activation that results in increased tumor cell killing and decreased tumor size [70,71].
Taken together, it is evident that autophagy plays a complex dual role in cancer development. Autophagy acts as a tumor suppressor during the early stage of tumorigenesis but promotes tumor malignancy and progression in existing tumors. Autophagy in both the tumor cells themselves and the host as well as the surrounding microenvironment promotes tumorigenesis and cancer progression.
ROLE OF P62 AND NRF2 IN LIVER TUMORIGENESIS
p62 is considered a cargo adaptor for selective autophagy due to its ability to bind directly to LC3 via its LC3 interaction region (LIR) [72,73]. However, more evidence now supports that p62 also serves as a multifunctional signaling hub within the cell [74-76]. In response to stress, p62 can bind to various partners through its multiple domains that are involved in various signaling pathways [75,77]. For example, the Phox/Bemp1 (PB1) domain at the N terminal of p62 can self-oligomerize to form homo-oligomers and hetero-oligomers, and can interact with the atypical protein kinase Cζ (PKCζ) [78,79]. The ZZ zinc finger region, and TB domains interact with multiple proteins that are related to p62-mediated nuclear factor-κB (NF-κB) activation [75]. The region between the ZZ and TB domain binds to Raptor, a subunit of the mTORC1 complex, leading to mTORC1 activation [80]. The LIR domain at the C terminal region of p62 binds to LC3 and triggers the selective-autophagy pathway [72,75]. Moreover, the KIR domain next to the LIR domain binds to Keap1 and transports it into the autophagosome for degradation, which leads to the activation of the non-canonical Keap1-Nrf2 pathway [81-84]. Both accumulated p62- and Keap1-positive aggregates have been found in autophagy-deficient mice (L-atg7 KO mice), causing persistent activation of Nrf2 and increasing the expression of Nrf2 target genes [22]. In addition, the ubiquitin-associated (UBA) domain binds to both mono- and polyubiquitinated proteins, which may explain why p62 is often found in protein aggregates and inclusion bodies [75,85].
As discussed above, p62 accumulation in the liver occurs in various types of liver diseases, including HCC [86-89], intrahepatic cholangiocarcinoma (CCA) [90], HCV and HBV infection [89,91], AALD [92], and non-alcoholic steatohepatitis [93,94]. Ectopic expression of p62 in mouse liver is sufficient to induce HCC in mice [87]. Moreover, loss of p62 in the human HCC cell line abrogates anchorage-independent growth, and whole-body depletion of p62 in mice restricts the size of hepatocellular adenomas in L-atg7 KO mice and L-tsc1 KO mice [21,22,87]. The mechanism by which hepatic p62 promotes HCC is generally thought to be due to its activation of Nrf2 via the noncanonic p62-Keap1-Nrf2 pathway, of which phosphorylated p62 further strengthens the binding affinity between p62 and Keap1 for Nrf2 activation [21,22,87,89]. Indeed, we found that deletion of Nrf2 in L-atg5 KO mice abolished hepatomegaly, liver injury and liver tumorigenesis [23]. Tumors grow in a relatively hash environment, often associated with hypoxia and limited nutrients, Nrf2 activation promotes the expression of genes for detoxification and antioxidant enzymes [95]. Moreover, p62-mediated Nrf2 activation also directs glucose to the glucuronate pathway, and glutamine towards glutathione synthesis which provides HCC cells with tolerance to anti-cancer drugs and increases their ability to proliferate [89].
In great contrast to p62’s role in hepatocytes, p62 in hepatic stellate cells (HSC) directly interacts with vitamin D receptor (VDR) and retinoid X receptor (RXR) to promote their heterodimerization resulting in the inhibition of HSC activation [88]. Whole body and HSC-specific p62 KO mice have increased HSC activation, enhanced liver inflammation and fibrosis, as well as HCC progression in response to high fat diet and hepatic carcinogen DEN [88]. Furthermore, decreased p62 protein levels have been observed in human HCC stroma cells. These findings suggest that p62 may have a cell-type specific role in HCC development. In hepatocytes, p62 may promote HCC development via Nrf2 activation whereas p62 in HSC may inhibit HSC activation and HCC progression via activation of VDR and RXR. Therefore, for clinical treatment purposes it is important to monitor p62 expression in different cell types. However, it remains a challenge to design cell type specific p62 targeting therapies for treating HCC.
As discussed above, genetic deletion of HMGB1 or Yap improved pathogenesis and tumorigenesis but did not eliminate tumor development in hepatic autophagy deficient mice [33,34]. However, deletion of Nrf2 completely abolished tumorigenesis in L-atg5 KO and L-atg7 KO mice [23,33]. These findings suggest that persistent activation of Nrf2 plays a more critical role than HMGB1 and Yap in autophagy-deficiency-induced liver tumorigenesis. Inhibition of Nrf2 may be a promising approach for treating HCC.
ROLE OF TFEB IN LIVER TUMORIGENESIS
The lysosome is the terminal component of autophagy and contains more than 50 acid hydrolases. Recent evidence suggests that transcriptional regulation of genes for lysosomal biogenesis and autophagy plays a critical role in autophagy [96]. Transcription factor EB (TFEB) is a basic helix-loop-helix leucine zipper transcription factor belonging to the microphthalmia/transcription factor E (MiT/TFE) family of transcription factors, which can bind to a specific gene sequence called the coordinated lysosomal expression and regulation (CLEAR) gene network motif [96]. TFEB is a master regulator for transcription of lysosomal biogenesis and autophagy genes [97,98]. TFEB is mainly regulated at the posttranslational level via phosphorylation of specific amino acid residues. Several kinases including the extracellular signal-regulated kinase 2 (ERK2) [98], mTORC1 [97], AKT [99], GSK3β [100], and protein kinase Cβ (PKCβ) [96] have been reported to phosphorylate TFEB. TFEB is phosphorylated at Ser142 by ERK2 and at both Ser142 and Ser211 by mTORC1, which promotes TFEB binding with the cytosolic chaperone 14-3-3 and sequesters TFEB in the cytosol, resulting in its inactivation [96,98]. In addition, STIP1 homology and U-box containing protein 1 (STUB1), a chaperone-dependent E3 ubiquitin ligase, interacts with phosphorylated TFEB resulting in TFEB ubiquitination and proteasomal degradation [101]. Conversely, calcineurin, a Ca2+-dependent phosphatase, dephosphorylates TFEB and increases TFEB nuclear translocation and activation [102]. In addition to phosphorylation and ubiquitination, TFEB can also be regulated by acetylation. General control non-repressed protein 5 (GCN5), a histone acetyltransferase, acetylates TFEB at multiple sites resulting in decreased TFEB transcription activity and lysosomal biogenesis [103]. In contrast, SIRT1, an NAD+-dependent deacetylase, deacetylates TFEB at lysine 116 leading to increased TFEB-mediated lysosomal gene expression [104].
TFEB and other members of the MiT-TFE family of transcription factors exhibit oncogenic features. Previous studies have revealed that the aberrant expression of several MiT-TFE family members is associated with different types of human cancers, such as renal cell carcinomas (RCC), alveolar sarcomas [105], non-small cell lung cancer, melanomas [106], and PDAC [59]. Chromosomal translocations and fusions of TFEB and TFE3 with other partner genes cause a particular type of RCC, referred to as translocation-RCC [107]. These fusions consistently preserve the TFEB/TFE3 open reading frame and always include the DNA-binding domains. While a variety of genes can be fused to TFEB or TFE3, a major consequence of the translocation with respect to oncogenic activity is a massive increase in the expression levels of TFEB or TFE3 protein [108,109]. In addition, altered TFEB expression and/or activity is associated with PDAC and non-small cell lung cancer, where they appear to support tumor growth via the induction of autophagy [59]. Interestingly, tumors in which MiT-TFE genes are amplified or overexpressed show an induction of RagD, a direct transcriptional target of TFEB, that resulted in mTORC1 hyperactivation [110]. The RagD GTPase is important for efficient mTORC1 recruitment to the lysosomal surface [111]. Silencing of MiT-TFE genes in primary cultured cells obtained from tumors resulted in a significant reduction of the hyperproliferative phenotype. Furthermore, xenotransplantation experiments performed using a melanoma cell line showed that silencing of RagD significantly reduced tumor growth [110]. These findings suggest that one of the mechanisms accounting for the oncogenic function of TFEB is likely due to RagD-mediated mTORC1 activation.
Birt-Hogg-Dubé syndrome is characterized by benign skin tumors, pulmonary and kidney cysts and RCC, which is caused by mutations in the tumor suppressor gene folliculin (FLCN) [112]. FLCN and its interacting partner protein FNIP2 act as a positive component of the mTORC1 pathway by promoting the binding of mTORC1 to the Rag heterodimer via its GTPase-activating protein (GAP) activity for RagC and RagD resulting in mTORC1 activation in cultured cells [113]. However, loss of FLCN in kidney cancers leads to hyperactivation of mTORC1 [114]. In a recent study, using a kidney-specific FLCN KO mouse model that mimics Birt-Hogg-Dubé syndrome, Napolitano et al found that TFEB is constitutively activated despite the hyperactivity of mTORC1 in kidney-specific FLCN KO mice [115]. They further found that mTORC1 can phosphorylate different substrates via a substrate-specific mechanism, which is mediated by Rag GTPases. Unlike other substrates of mTORC1, such as S6K and 4E-BP1, TFEB is strictly dependent on the amino-acid-mediated activation of RagC and RagD GTPases but is insensitive to Ras homologue enriched in brain (Rheb) activity induced by growth factors. Depletion of TFEB in kidneys of FLCN KO mice fully normalized mTORC1 activity and rescued the disease phenotype, suggesting activation of TFEB is the major driver for kidney cysts and cancer in FLCN KO mice [115].
Despite the above-mentioned evidence linking TFEB to several cancers, whether TFEB inhibits or promotes HCC remains elusive. Notably, TFEB activity is impaired in both AALD and NALFD [14,116,117], two risk factors for HCC, implicating a potential role of TFEB in HCC. The liver is the most active metabolic organ and is frequently exposed to xenobiotics and metabolites that are often toxic which can lead to acute or chronic liver injury. To deal with various toxic insults, the liver has evolved a remarkable regenerative capacity to help with recovery from injury [118]. The high regeneration capacity is largely attributable to the ability of its differentiated epithelial cells, hepatocytes and biliary epithelial cells, to proliferate after injury. However, when the proliferation of one subtype of cells in the liver is compromised and unable to make its own histologic recovery, the other cell types will step in and transdifferentiate to the lost cell type [118]. While it remains to be a controversial hot topic, increasing evidence indicates that hepatocytes can trans-differentiate to cholangiocytes in response to biliary injury and cholangiocytes can trans-differentiate to hepatocytes for the loss of injured hepatocytes [119,120].
In a recent study, Pastore et al reported that TFEB can drive liver progenitor cell (LPC) differentiation and promote the formation of ductular structures [121]. Among the different cell types in the liver, TFEB is highly expressed in biliary cells. TFEB drives the differentiation of LPC into the progenitor/cholangiocyte lineage while inhibiting hepatocyte differentiation during the mouse liver development or upon regeneration in response to injury. Tfeb transgenic mice exhibit an increase in the number of bile ductules after injury whereas L-tfeb KO mice exhibit a decrease. Transcriptomic and CHIP studies show that Sox9, a marker of precursor and biliary cells, is a direct TFEB target and a primary mediator of its effects on liver cell fate. Interestingly, results from lineage tracing experiments show that overexpression of TFEB in hepatocytes can promote trans-differentiate to cholangiocytes. Tfeb transgenic mice at 6-months-old either die or have to be euthanized due to poor health conditions. These mice have increased fibrosis and develop liver cysts which mimic a polycystic liver and develop CCA-like phenotype [121]. These findings suggest that TFEB may play a crucial role in determining liver cell fate during development and regeneration, and may also contribute to CCA, which seems independent of its functions in lysosomal biogenesis.
ONCOGENES AND TUMOR SUPPRESSORS
In general, oncogenes such as class-I phosphatidylinositol 3-kinase (PI3K) and AKT suppress autophagy via activating mTORC1 whereas tumor suppressor genes such as tsc1, pten, lkb, and ampk activate autophagy through inhibition of mTORC1 (Fig. 1) [122]. Class I- PI3Ks are activated by growth factor receptor tyrosine kinases (RTKs) that catalyze the formation of lipid secondary messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) from phosphatidylinositol-4,5-bisphosphate (PIP2). PIP3 then binds with AKT via its pleckstrin homology (PH) domain to recruit AKT to the plasma membrane, where AKT is activated by 3-phosphoinositide dependent kinase (PDK1) at threonine 308 and serine 473 by the rapamycin-insensitive mTORC2 [123,124]. The tumor suppressor PTEN reverses the effects of PI3K by dephosphorylating PIP3 which in turn inhibits AKT activation. However, loss of function mutations in PTEN results in AKT activation in a wide spectrum of human cancers [125]. Activated AKT directly phosphorylates TSC2 and inactivates TSC1-TSC2 complex, which has GAP activity towards the small G-protein Rheb, and in turn activates mTORC1 [126,127]. In contrast, the AMP-activated protein kinase (AMPK) phosphorylates TSC2 and enhances TSC1-TSC2 activity resulting in mTORC1 inhibition in response to energy starvation [128]. Alternatively, AMPK can be phosphorylated and activated by serine-threonine kinase liver kinase B1 (LKB1) [129,130], a tumor suppressor gene that is responsible for the inherited cancer disorder Peutz-Jeghers syndrome [131]. Together, AMPK negatively regulates mTORC1 to trigger the catabolic autophagy process to generate more energy substrates, whereas, AKT positively regulates mTORC1 to promote the anabolic process and shutdown autophagy. L-pten KO mice have increased AKT and mTORC1 activation, impaired hepatic autophagy and develop hepatomegaly, steatohepatitis and spontaneous HCC [132,133]. L-tsc1 KO mice have constitutive mTORC1 activation, impaired autophagy and develop spontaneous HCC [134]. Therefore, oncogenes inhibit whereas tumor suppressor genes activate autophagy resulting in either the promotion or suppression of HCC in the liver.
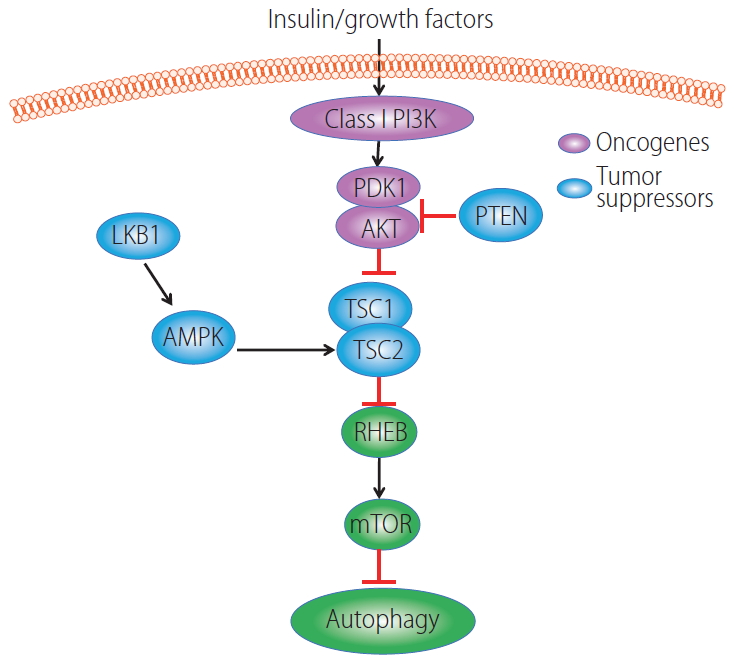
Regulation of autophagy by oncogenes and tumor suppressor genes. In the presence of insulin or growth factors, AKT is activated due to increased PI3K activity and binding with PDK1. AKT is negatively regulated by PTEN. Activated AKT phosphorylates TSC2 resulting in decreased TSC1-TSC2 complex activity leading to enhanced GTP-bound form of Rheb to stimulate mTORC1 activation. LKB1 phosphorylates and activates AMPK, and activated AMPK increased TSC2 phosphorylation on a different site of AKT resulting in increased TSC1-TSC2 complex activity which leads to decreased mTORC1 activation. Increased mTORC1 activity negatively regulates autophagy. PI3K, phosphatidylinositol 3-kinase; PDK1, phosphoinositide dependent kinase; AKT, protein kinase B; LKB1, serine-threonine kinase liver kinase B1; PTEN, phosphatase and tensin homolog; AMPK, AMP-activated protein kinase; TSC, tuberous sclerosis complex; RHEB, Ras homologue enriched in brain; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1.
SUMMARY
Since the identification of the first Atg in yeast in the 1990s, significant progress has been made in the understanding of the molecular mechanisms underlying the process of autophagy as well as the vital physiological role of autophagy in human diseases. In regards to the role of autophagy in cancer, it is now clear that autophagy exerts both tumor-suppressive and tumor-promoting roles in a context dependent manner. In the early phase of tumor initiation, autophagy acts as a tumor suppressor by removing damaged organelles and proteins to guard against genome instability. In the late phase of tumor development and progression, transformed cancer cells and stromal cells use autophagy to generate nutrients within the hash tumor microenvironment to increase their chances of survival. Viral infection, AALD and NAFLD, all lead to impaired autophagy and promotion of HCC. Therefore, different approaches to manipulate autophagy should be considered for the prevention/treatment of HCC. For preventative purposes, pharmacological activation of autophagy to correct virus or alcohol and diet-impaired hepatic autophagy may be beneficial. However, for treating existing HCC, pharmacological inhibition of autophagy or Nrf2 in combination with other chemotherapy drugs should be a more efficient strategy.
Acknowledgements
This work was partially supported by NIH grants: R37 AA020518, R01 DK102142, U01 AA024733 and P20GM103549 & P30GM118247.
Notes
Authors’ contributions
Conceptualization, W.X.D., X.J.C., and H.Q.; Writing and Original Draft Preparation, W.X.D., X.J.C., H.Q., and S.G.W., Writing, Review and Editing, W.X.D., X.J.C., H.Q., S.G.W., and S.F.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
AALD
alcohol associated liver disease
AKT
protein kinase B
AMPK
AMP-activated protein kinase
ARG1
arginase I
ASS1
argininosuccinate synthase
CCA
cholangiocarcinoma
CLEAR
coordinated lysosomal expression and regulation
CSC
cancer stem cell
DAMP
damage-associated molecular pattern
DEN
diethylnitrosamine
ER
endoplasmic reticulum
ERK2
extracellular signal-regulated kinase 2
FLCN
folliculin
GAP
GTPase-activating protein
GCN5
general control non-repressed protein 5
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HMGB1
high mobility group box 1
HSC
hepatic stellate cells
KO
knockout
LIR
LC3 interaction region
LKB1
serine-threonine kinase liver kinase B1
LPC
liver progenitor cell
MHC
major histocompatibility complex
MiT
microphthalmia
mTOR
mechanistic target of rapamycin
mTORC1
mechanistic target of rapamycin complex 1
NAFLD
nonalcoholic fatty liver diseases
NANOG
Nanog homeobox
NF-κB
nuclear factor-κB
Nrf2
nuclear factor (erythroid-derived 2)-like 2
PB1
phox/bemp1
PDAC
pancreatic ductal adenocarcinoma
PDK1
phosphoinositide dependent kinase
PH
pleckstrin homology
PI3K
phosphatidylinositol 3-kinase
PINK1
PTEN-induced kinase 1
PIP2
phosphatidylinositol-4,5-bisphosphate
PIP3
phosphatidylinositol-3,4,5-trisphosphate
PKC
protein kinase C
PTEN
phosphatase and tensin homolog
RCC
renal cell carcinomas
Rheb
ras homologue enriched in brain
RTKs
receptor tyrosine kinases
RXR
retinoid X receptor
STUB1
STIP1 homology and U-box containing protein 1
TEAD
transcriptional enhanced associate domain
TFE
transcription factor E
TFEB
transcription factor EB
TSC
tuberous sclerosis complex
UBA
ubiquitin-associated
VDR
vitamin D receptor
Yap
yes-associated protein
