Hepatoid adenocarcinoma of the stomach: an unusual case of elevated alpha-fetoprotein with prior treatment for hepatocellular carcinoma
Article information
Abstract
Hepatoid adenocarcinoma (HAC) is a rare type of extrahepatic carcinoma whose morphology is similar to that of hepatocellular carcinoma (HCC). Metachronous HCC and HAC in the same patient is extremely rare. The case of a 68-year-old man with chronic hepatitis B infection who had both HCC and HAC of the stomach is reported herein. Nine years previously this patient had been diagnosed with HCC and received a right lobectomy. HCC that recurred at the caudate lobe at 6 months after the operation was successfully treated with transarterial chemoembolization. The patient was followed up regularly thereafter without evidence of tumor recurrence for 9 years. In July 2010 his serum alpha-fetoprotein (AFP) level elevated from 6.5 ng/mL to 625.4 ng/mL, and he developed a probable single metastatic lymph node around the hepatic artery without intrahepatic lesions. Subsequent evaluation with upper endoscopy revealed a 4-cm ulcerative lesion on the antrum of the stomach. Subtotal gastrectomy was performed with lymph-node dissection. Histologic examination revealed a special type of extrahepatic AFP-producing adenocarcinoma-HAC with lymph-node metastasis-which indicates that HAC can be a cause of elevated AFP even in patients with HCC. HAC should be considered if a patient with stable HCC exhibits unusual elevation of AFP.
INTRODUCTION
Hepatoid adenocarcinoma (HAC) is a type of extrahepatic carcinoma, which has a similar morphology to hepatocellular carcinoma (HCC).1 The first HAC case was described by Bourrelille et al. in 1970.2 It occurs in many different organs such as the lung, pancreas, ovary, and gastric tissue.3 Elevated alpha-fetoprotein (AFP) level is a characteristic finding of HAC.4 In patients at a high risk for HCC, such as patients with cirrhosis, chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, elevated serum AFP level is usually indicative of HCC. If HAC occurs in patients at a high risk for HCC, correct diagnosis of HAC can be a challenging issue to clinicians. Recently, HAC cases with liver metastasis mimicking HCC have been reported in many countries.1,5,6 However, a case of a metachronous HCC and HAC is very rare. Here we report an unusual case of HAC of the stomach which was diagnosed in a patient who had HCC.
CASE REPORT
A 68-year-old man was evaluated for elevated serum AFP level. He had been diagnosed with chronic HBV infection and cirrhosis twenty years ago. He was receiving antiviral treatment with oral antiviral agents (lamivudine and adefovir dipivoxil) from 2003 to 2008 due to HBV reactivation with elevated liver enzyme, positive for HBeAg and HBV DNA of 199.1 pg/mL. Tests for HCV and human immunodeficiency virus (HIV) were negative. After the administration of antiviral agents, liver enzyme was normalized and HBeAg seroconversion occurred. In December 2001, ten years prior to this evaluation, an arterial enhancing lesion approximately 3 cm in size with delayed washout at the segment 6/7 area of the liver (Fig. 1A, B), along with elevated serum AFP level (7,463 ng/mL), was found. The patient was diagnosed with HCC and treated with a right lobectomy, which revealed Edmondson grade II HCC (Fig. 1C, D). Six months after the liver resection, a 1.5 cm sized enhancing nodule with delayed washout was found in the caudate lobe. In August and October 2002, two times of transarterial chemoembolization were performed at the lesion and follow-up computed tomography (CT) showed complete lipiodol uptake at the lesion. Serum AFP had returned to normal level (4.8 ng/mL) and since then, he had been regularly followed up for nine years without evidence of tumor recurrence.
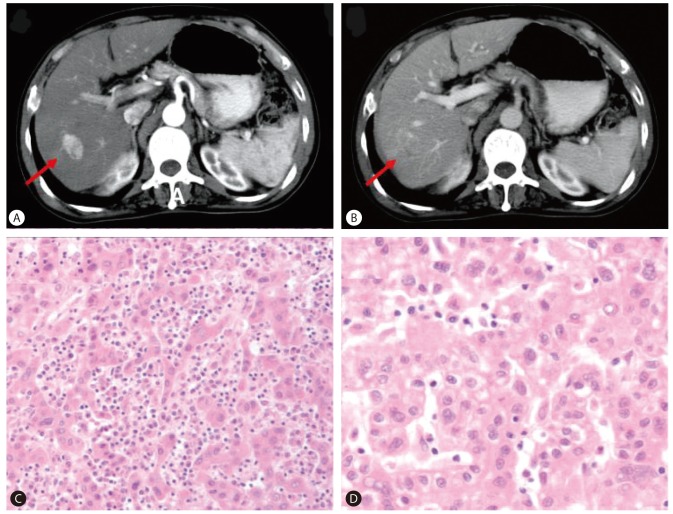
(A, B) Liver computed tomography (CT) scan showed a 3-cm arterial enhancing lesion with delayed washout at segment 6/7 of the liver (A, arterial phase; B, portal phase). (C, D) Tumor in the liver showed Edmondson grade II HCC (C: H&E, ×100, D: H&E, ×400).
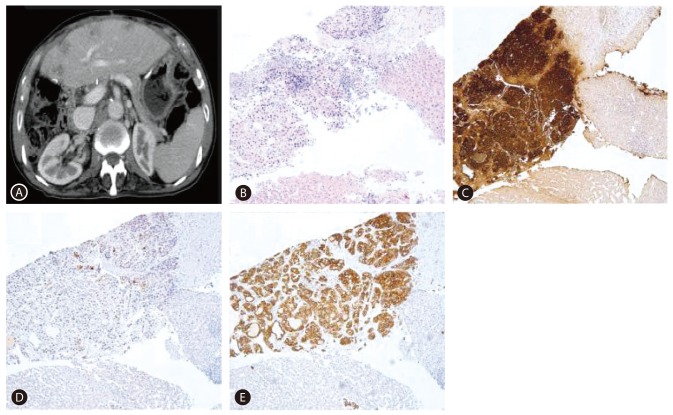
In July 2010, elevation of serum AFP level was noticed (from 6.5 ng/mL to 625.4 ng/mL during a period of six months). At this point, he had no specific symptoms or physical findings. A white blood cell count was 3,990/mm3, a hemoglobin level was 14.6 g/dL, a platelet count was 73,000/mm3, a serum protein level was 7.7 g/dL, a serum albumin level was 3.8 g/dL, a serum bilirubin level was 1.5 mg/dL, a serum aspartate aminotrasferase level and a serum alanine aminotransferase level were 56 IU/L and 57 IU/L, a serum creatinine level was 0.84 mg/dL, and a prothrombin time (%) was 81%. Other tumor markers were within normal range (serum proteins induced by vitamin K absence or antagonist-II (PIVKA-II): 23 mAU/mL, carcinoembryonic antigen (CEA): 1.8 ng/mL and carbohydrate antigen 19-9 (CA 19-9): 9.2 IU/mL). Liver CT revealed a 2.5 cm low-attenuated nodular lesion around the common hepatic artery, suggesting metastatic lymphadenopathy without intrahepatic lesion. Positive emission tomography (PET)-CT found a hypermetabolic lymph node (p-SUV=4.1) at the common hepatic artery area, but no other fluorodeoxyglucose (FDG) uptake that suggested the presence of a malignant tumor was found. Based on elevated AFP level and prior history of HCC, metastatic lymphadenopathy from HCC was the differential diagnosis. Radiation therapy for regional lymph node was planned, and he underwent upper endoscopy for screening purposes before receiving radiation therapy. Incidentally, a 4.0 cm ulcerative lesion at the lesser curvature side of the antrum was found during an upper endoscopy (Fig. 2A). Gastric biopsy showed poorly differentiated tubular adenocarcinoma. The patient underwent Billroth type II subtotal gastrectomy with D2 lymph node dissection. Microscopic examination revealed HAC with lymph node metastasis as immunohistochemical (IHC) study showed that tumor cells were positive for AFP and polyclonal CEA (Fig. 2B, C, D, E).
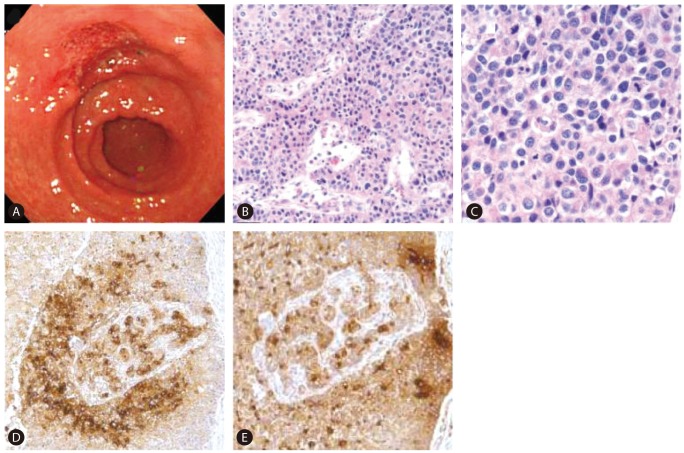
(A) Upper endoscopy revealed a 4-cm ulcer on the lesser curvature side of the antrum of the stomach. It is morphologically suspicious of early gastric cancer type III. (B-E) Tumor in the stomach showed an HAC. It was immunohistochemically positive for AFP and polyclonal carcinoembryonic antigen (CEA) (B: H&E, ×100; C: H&E, ×400; D: AFP, ×100; E: polyclonal CEA, ×100).
After the operation, serum AFP level decreased, and he received titanium silicate (TS)-1 for adjuvant chemotherapy. However, 6 months after the gastrectomy, multiple small hepatic nodules were found in the liver during adjuvant chemotherapy (Fig. 3A). A liver biopsy was taken and HAC was diagnosed as IHC positive for AFP and CK19, focally positive for CEA, whereas negative for CK7 and CK20 (Fig. 3B, C, D, E). He was then switched to palliative chemotherapy with carpecitabine and cisplatin (XP regimen). After 4 months (four cycles) of chemotherapy, a CT scan revealed a progression of liver metastasis and newly developed pulmonary metastasis. He was put under the second-line palliative chemotherapy with fluorouracil, leucovorin and irinotecan (FOLFIRI regimen). He is currently being followed up for 21 months.
DISCUSSION
A case of HAC which developed nine years after the treatment of HCC in one patient is extremely rare. Morinaga et al6 described a case in which the patient was HCV seropositive, had high serum AFP level and was confirmed to have liver masses. Autopsy revealed the coexistence of primary HCC and HAC of the stomach with multiple liver metastases. Lauffer et al7 reported a case of a patient with HCC as well as HAC of the stomach without liver metastasis. To our knowledge, only two cases described above have reported concurrent HCC and HAC, and this is the first case of metachronous HCC and HAC in a patient.
AFP is a tumor marker variably secreted by HCC and is widely used in clinical practice as a diagnostic tool. Serial measurement of serum AFP level is used for HCC surveillance in high risk individuals, and it is also used in post-HCC treatment assessment.8 Changes in serum AFP level help to assess treatment response, and also help to identify recurrence of HCC. However, AFP is not HCC specific. Elevation in the serum AFP level can be found in cases of pregnancy, benign liver disease associated with liver regeneration such as hepatitis and cirrhosis, tumors of gonadal origin (both germ cell and non-germ cell) and variety of gastrointestinal malignancies as well as in HAC.9 HAC is an AFP-producing malignant tumor that may be found in several organs. The stomach is the most common site affected.3 Ishikura et al10 proposed the term 'hepatoid adenocarcinoma of the stomach' as an AFP-secreting gastric carcinoma with features of hepatic differentiation in 1985. The reported incidences of AFP-producing gastric adenocarcinoma have been from 1.3%-15% of all gastric cancers.11
The pathogenesis of HAC is not fully understood but several features have revealed. This tumor shows a high angioinvasiveness and this feature shared with epithelial neoplasm such as HCC and renal cell carcinoma.4 One hypothesis might be that hepatoid cells are more proximal to the totipotent cells, such as fetal cells, from an ontogenetic standpoint, that liver specific genes normally in the repressed state may be activated during the process of carcinogenesis, expressing cells with a hepatic phenotype.12 These assumptions need to be proven and more studies about pathogenesis and biology of this tumor are required.
Clinically, this neoplasm is characterized by a predilection for older age, aggressive clinical course, and poor prognosis because of its extensive hematogenous metastasis to the liver and early metastasis to regional lymph nodes.4,11,13,14 Inagawa et al8 reviewed 85 cases of hepatoid adenocarcinoma of stomach that have been reported. Early-stage cancer was seen in only 11 patients; the had the were advanced stage. Most of the reported patients preoperatively diagnosed with metastases to the liver or lymph nodes. Furthermore, most of them died within 2 years of the surgery, despite systemic chemotherapy having been administered in several patients. One possibility of poor prognosis is AFP has a suppressive effect on lymphocyte transformation.15 Alpha-1 antitrypsin and antichymotripsin, produced by HAC, have immunosuppressive and protease-inhibitory properties that enhance invasiveness.16
Diagnosis of HAC depends on recognition of the histological feature. The histological finding reveals glandular adenocarcinoma with hepatoid foci and IHC study affords more special support.14,17 Terracciano et al8 reported differences in reaction patterns of HAC by IHC and molecular study of eight cases. All HACs showed positive in AFP and seven out of eight cases were positive for polyclonal CEA. On the other hand, in 121 HCC cases, positivity for AFP and polyclonal CEA was 8.2% and 67.7%, respectively. IHC positivity of CK19 and CK20 was 8.2% and 1.6% in HCC, 94% and 47% in HAC, respectively. CK7, which is highly positive for combined hepatocellular-biliary carcinoma, showed negative in all eight cases. In short, positivity of virtually all HACs for AFP and canalicular staining for polyclonal CEA underline its hepatoid nature. On the other hand, positivity for CK19 and CK20 and negativity for hepatocyte paraffin 1 (HepPar1) and CK7 were distinctive features of HAC.
Most HAC cases show elevation in the AFP level, thus AFP elevation in patients may indicate HAC as in the case presented in our study. The elevation of serum AFP level should be interpreted in context of the clinical situation. There can be various reasons for AFP elevation, and this case demonstrates the importance of differential diagnosis in interpreting elevated AFP level. The patient experienced an increase in serum AFP level at two different times. On the first observation, the AFP elevation was due to HCC while the second observed AFP elevation was due to HAC of the stomach. Since this patient was among high risk patients for HCC (chronic HBV infected patients who had prior treatment for HCC), elevation in serum AFP levels were indicative for HCC recurrence. Notably, this patient had no intrahepatic lesion, and a single lymphadenopathy was found. He received upper endoscopy because screening endoscopy was a routine procedure for patients planned to undergo radiation therapy in our center, and incidentally, the detected gastric lesion was later confirmed as HAC. This case demonstrated that HAC should be included in a differential diagnosis for patients with elevated AFP level. Since the stomach is the most frequently affected site for HAC and regional lymph node metastasis frequently occurs, evaluation with upper endoscopy is recommendable to screen for HAC of the stomach in patients with elevated AFP, lymphadenopathy, but no intrahepatic lesion.
In summary, we describe an unusual case with both HCC and HAC. The elevation of AFP was due to HCC on initial observation, but was due to HAC for the second spike in AFP. HAC was diagnosed incidentally, as elevation of AFP was considered as an indicative of HCC. A differential diagnosis of HAC should be considered even for a patients at a high risk for HCC in cases of serum AFP elevation.
Notes
The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
CA 19-9
carbohydrate antigen 19-9
CEA
carcinoembryonic antigen
CT
computed tomography
FDG
fluorodeoxyglucose
HAC
hepatoid adenocarcinoma
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HIV
human immunodeficiency virus
IHC
immunohistochemical
PET
positive emission tomography
TS
titanium silicate