Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study
Article information
Abstract
Background/Aims
Hypothyroidism is reported to contribute to the development of nonalcoholic fatty liver disease (NAFLD). We compared the risk of the development of NAFLD among three groups with different thyroid hormonal statuses (control, subclinical hypothyroidism, and overt hypothyroidism) in a 4-year retrospective cohort of Korean subjects.
Methods
Apparently healthy Korean subjects without NAFLD and aged 20-65 years were recruited (n=18,544) at health checkups performed in 2008. Annual health checkups were applied to the cohort for 4 consecutive years until December 2012. Based on their initial serum-free thyroxine (fT4) and thyroid-stimulating hormone (TSH) levels, they were classified into control, subclinical hypothyroidism (TSH >4.2 mIU/L, normal fT4), and overt hypothyroidism (TSH >4.2 mIU/L, fT4 <0.97 ng/dL) groups. NAFLD was diagnosed on the basis of ultrasonography findings.
Results
NAFLD developed in 2,348 of the 18,544 subjects, representing an overall incidence of 12.7%: 12.8%, 11.0%, 12.7% in the control, subclinical hypothyroidism, and overt hypothyroidism groups, respectively. The incidence of NAFLD did not differ significantly with the baseline thyroid hormonal status, even after multivariate adjustment (subclinical hypothyroidism group: hazard ratio [HR]=0.965, 95% confidence interval [CI]=0.814-1.143, P=0.67; overt hypothyroidism group: HR=1.255, 95% CI=0.830-1.899, P=0.28).
Conclusions
Our results suggest that the subclinical and overt types of hypothyroidism are not related to an increased incidence of NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is characterized by excessive hepatic accumulation of triglycerides and free fatty acids in the liver.1 The incidence of NAFLD is increasing rapidly, and it is the most common cause of abnormal liver function results worldwide.2 The increase in the prevalence of NAFLD has been attributed to the global increase in the prevalence of obesity and other metabolic risk factors. Advanced age and metabolic disorders such as type 2 diabetes mellitus, impaired glucose tolerance, and central obesity, are among the risk factors for NAFLD.3 Cryptogenic cirrhosis is a term used for patients with liver cirrhosis who lack any identifiable viral, alcoholic, autoimmune or drug-related causes for the condition. Many clinicians now believe that a considerable number of these patients have cirrhosis due to nonalcoholic steatosis hepatitis (NASH).4
NAFLD has broad pathological spectrum, ranging from simple steatosis to NASH, and potentially progressing to fibrosis and cirrhosis.5 After fat infiltrates the liver, progression to hepatocellular inflammation and fibrosis may occur.
The thyroid gland is significantly involved in energy homeostasis, lipid and carbohydrate metabolism, regulation of body weight and adipogenesis. Subclinical and overt hypothyroidism has been associated with metabolic syndrome, cardiovascular mortality and disturbance in lipid metabolism.6
Several clinical studies have investigated the role of hypothyroidism as a predictor for NAFLD in cross-sectional analyses,78910 but the results have been contradictory. No longitudinal study has demonstrated a relationship between hypothyroidism and NAFLD.
Therefore, in this study, we investigated the relationship between baseline thyroid hormonal status and the incidence of NAFLD in a 4-year follow-up of a cohort of 18,544 apparently healthy Korean subjects.
MATERIALS AND METHODS
1. Participants
This was a retrospective cohort study, and the subjects were participants in the Kangbuk Samsung Health Study, a large database of participants in a medical health check-up program at the Health Promotion Center of Kangbuk Samsung Hospital. The purpose of the medical health check-up program is to promote the health of employees through regular health check-ups and to enhance early detection of disease. Most of the examinees were employees of various companies. The cost of the medical examinations is largely paid for by employers, and a considerable proportion of the examinees undergo examinations.
Initial data were obtained from 28,861 subjects in whom annual health check-ups were performed for 5 consecutive years between January 2008 and December 2012. Among these subjects, 7,317 were excluded (5,835 due to initial fatty liver disease, 1,266 due to positive serological marker for chronic viral hepatitis, 1,436 were unclassified by thyroid hormonal status, and 1,780 consumed alcohol more than 20 gram per day (Fig. 1).
Finally, 18,544 apparently healthy subjects without NAFLD aged 20-65 years were included. Questionnaires were used to determine alcohol consumption (gram per day). Alcohol intake and smoking habits were assessed by chart review and a standardized questionnaire. Based on initial serum free thyroxine (fT4) and thyroid-stimulating hormone (TSH), the subjects were classified into euthyroid control [TSH 0.27~4.2 mIU/L, fT4 0.97~1.68 ng/dL], subclinical hypothyroidism [TSH >4.2 m IU/L, fT4 0.97~1.68 ng/dL] and overt hypothyroidism [TSH >4.2 m IU/L, fT4<0.97 ng/dL] groups. NAFLD was diagnosed based on the results of abdominal ultrasonography, after excluding heavy alcohol consumption, and viral, or other liver diseases.
This study protocol was approved by the Institutional Review Board and Ethnics Committee of Kangbuk Samsung Hospital. The participants provided written informed consent for their medical check-up data to be used in this study.
2. Biochemical assays
Blood samples were obtained after a 12 hour overnight fast and used to measure fasting plasma glucose (FBG), total cholesterol, low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol (HDL), fasting insulin, creatinine, direct bilirubin, and the following liver function parameters: aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), and alkaline phosphatase (ALP). As a marker of insulin resistance (IR), homeostatic model assessment (HOMA) of IR was calculated as follows : HOMA-IR=[fasting insulin(µIU/mL) × fasting glucose (mmol/L)]/22.5.11 Baseline thyroid function (free T4 and TSH levels) was measured using commercial immunoradiometric assays.
3. Ultrasonography
Abdominal ultrasonography (Logic Q700 MR, GE, Milwaukee, WI, USA) was performed in all subjects. Fatty liver was diagnosed based on standard criteria, including hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring, using a 3.5 MHz probe. Several experienced radiologists, who were blinded to the clinical status of the subjects, performed the ultrasounds. However, we did not assess inter-observer reliability.
4. Statistical analysis
Normality was tested using the Kolmogorov-Smirnov test. The chi-squared test was used to compare categorical variables between groups. For continuous variables, parameters that followed a normal distribution were analyzed with a t-test or analysis of variance (ANOVA) and described as the mean±SD. Parameters that did not follow a normal distribution were analyzed with the Mann-Whitney U test or the Kruskal-Wallis test and expressed as the median. Kaplan-Meier survival analyses were performed for incident NAFLD for 4 years according to baseline thyroid hormonal status. Cox proportional hazards regression models were used after adjusting for several confounders. Statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P<0.05.
RESULTS
The study cohort included 18,544 subjects. Among them, 2,348 (12.7%) developed NAFLD within 4 years. The median duration to develop NAFLD was 2.92 years. The subjects were divided into two groups for follow up, such as those who developed NAFLD (n=2,348) and those who did not (n=16,196). Table 1 shows a comparison of the baseline characteristics between the subjects according to the development of NAFLD. The NAFLD group was older than that of the non-NAFLD group (39.2±5.9 vs. 37.8±5.7 years). The NAFLD group had a significantly higher proportion of males, and a higher body mass index (BMI). Additionally, baseline serum levels of total cholesterol, triglycerides, LDL, FPG, fasting insulin, AST, ALT, blood urea nitrogen (BUN), and creatinine were significantly higher in the NAFLD group than those in the non-NAFLD group. However, TSH and free T4 were not associated with the development of NAFLD.

Baseline clinical characteristics of the subjects recruited in 2008 and grouped according to the development of nonalcoholic fatty liver disease (NAFLD) during 2009-2012
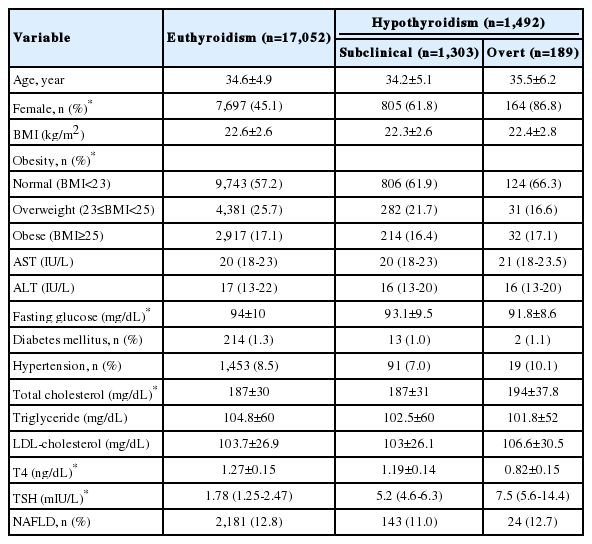
The subjects were divided into three groups according to initial thyroid hormonal status. (17,052 in the euthyroid control, 1,303 in the subclinical hypothyroidism, and 189 in the overt hypothyroidism groups). The clinical and laboratory characteristics of the subjects are shown in Table 2. Among them, 2,181 (12.8%) euthyroid subjects, 143 (11%) subclinical hypothyroidism subjects and 24 (12.7%) overt hypothyroidism subjects developed NAFLD during the 4 year of follow-up (Table 2). However, this result was not statistically relevant (P=0.132).
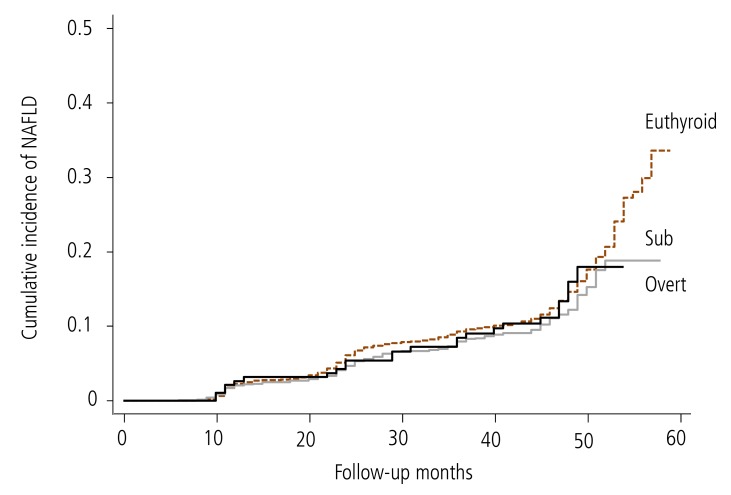
Cox proportional hazards regression analyses were used to estimate hazard ratios (HRs) for the incidence of NAFLD due to hypothyroidism during the follow-up. Our analysis showed that subclinical and overt hypothyroidism were not risk factors for developing NAFLD in the crude model (subclinical: HR, 0.847; 95% confidence interval [CI], 0.715-1.003; overt: HR, 0.968; 95 % CI, 0.647-1.447). Metabolic syndrome was strongly associated with this relationship. Therefore, we further adjusted the Cox proportional hazards regression analysis for indicators of metabolic syndrome. However, even after adjusting for sex, age, BMI, TGs and HDL, the relationship between subclinical and overt hypothyroidism and incident NAFLD was not significant. The adjusted HRs (95% CI) were 0.965 (0.814-1.143) and 1.255 (0.83-1.89), respectively (Table 3). The Kaplan-Meier survival curve (cumulative incidence of NAFLD) showed no difference among the three groups (P=0.15) (Fig. 2). These results indicate that hypothyroidism is not an independent factor predicting the development of NAFLD.
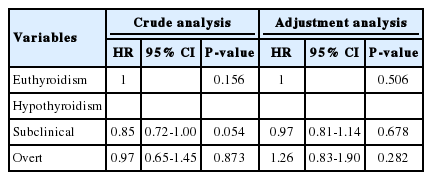
Cox proportional-hazards ratio analysis for the development of NAFLD during 2009-2012 grouped according to the thyroid hormonal status in 2008
DISCUSSION
We did not demonstrate an association between thyroid hormonal status and the incidence of NAFLD, and the incidence of NAFLD did not increase with hypothyroidism.
Several studies have investigated the association between hypothyroidism and NAFLD. Chung et al. showed that subclinical hypothyroidism, even in the range of upper normal TSH levels, is closely associated with NAFLD in a dose-dependent manner. However, a causal relationship between hypothyroidism and NAFLD was not detected in their cross-sectional study.7 In addition, three studies have indicated that hypothyroidism is an independent risk factor for developing NAFLD/NASH.8910 Several studies have reported that lower free T4 is an independent risk factor for NAFLD.71213 Several other studies have shown that increased serum TSH is an independent risk factor for NAFLD/NASH.131415 In contrast, similar to our finding, three studies could not find an association between hypothyroidism and NAFLD. Mazo et al. did not find an association between hypothyroidism, simple steatosis, and NASH.16 Ittermann et al. reported no association between hyper- or hypothyroidism and hepatic steatosis in a population-based study,12 and Eshraghian et al. showed that thyroid hormone abnormalities in patients with NAFLD may be due to changes in thyroid hormones due to other non-thyroid illnesses.17
An explanation for the association between hypothyroidism and NAFLD is that hypothyroidism is associated with metabolic syndrome. Several studies have reported that hypothyroidism is related to obesity and metabolic syndrome.181920 Thyroid hormones stimulate the expression of uncoupling proteins in the mitochondria of fat and skeletal muscle, through modulate adrenergic receptor numbers by enhancing responsiveness of catecholamine.21 Thus, thyroid hormones influence body weight, thermogenesis, lipolysis, and cholesterol metabolism. Patients with NAFLD have abnormal lipid profiles, such as elevated total cholesterol, LDL and TG levels.22 Among these, TGs is a one of metabolic syndrome components. Hypertriglyceridemia increases importation of TGs into the liver and is therefore associated with the development of NAFLD.23 For this reason, some studies have suggested that hypothyroidism may contribute to the development of NAFLD, or that it may be associated with other NAFLD risk factors.
However, we found no association between hypothyroidism with NAFLD, which conflicts with studies that have reported an association between hypothyroidism and NAFLD. One study suggested that thyroid hormone abnormalities in patients with NAFLD may occur be due to other non-thyroidal illnesses.17 This concept may explain the absence of the association between hypothyroidism and NAFLD observed in our study.
This study had some strength. Most of other studies have investigated the prevalence of NAFLD according to thyroid status (cross-sectional study), but we report the incidence of NAFLD through a 4 year follow-up (longitudinal cohort study). As most of our participants were healthy young people, we thought that the relationship between hypothyroidism and NAFLD would be less affected by other variables.
This study had several limitations. First, the NAFLD diagnosis was not histologically confirmed, after ultrasound, which has reported sensitivity of 67-89% and specificity of 77-89%. Second, alcohol intake was determined by a self-reported questionnaire; therefore, we cannot rule out the possibility of misreporting or recall bias. In addition, lifestyle risk factors, which can affect future development of NAFLD, including exercise and dietary habits, were not considered. Thus, the data were subject to potential under or over-estimation. Third, our cohort was consisted of relatively young people, so it may not represent general population. Fourth, our study excluded baseline NAFLD patients. This may bias the results, leading to underestimation of NAFLD incidence in the overall course of hypothyroidism. Lastly, our cohort was comprised of participants who had volunteered for health check-ups, which may have introduced selection bias.
In conclusion, although a high prevalence of hypothyroidism has been reported in with NAFLD, we found no significant correlation between hypothyroidism and the incidence of NAFLD. Further studies are needed to clarify the exact role of hypothyroidism in the progression of NAFLD.
Acknowledgements
No direct financial support was provided to conduct this study.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
fT4
free thyroxine
HOMA-IR
Homeostatic model assessment of insulin resistance
NAFLD
Nonalcoholic fatty liver disease
TGs
Triglycerides
TSH
Thyroid-stimulating hormone