Hepatitis B surface antigen titer is a good indicator of durable viral response after entecavir off-treatment for chronic hepatitis B
Article information
Abstract
Background/Aims
Clear indicators for stopping antiviral therapy in chronic hepatitis B (CHB) patients are not yet available. Since the level of hepatitis B surface antigen (HBsAg) is correlated with covalently closed circular DNA, the HBsAg titer might be a good indicator of the off-treatment response. This study aimed to determine the relationship between the HBsAg titer and the entecavir (ETV) off-treatment response.
Methods
This study analyzed 44 consecutive CHB patients (age, 44.6±11.4 years, mean±SD; men, 63.6%; positive hepatitis B envelope antigen (HBeAg) at baseline, 56.8%; HBV DNA level, 6.8±1.3 log10 IU/mL) treated with ETV for a sufficient duration and in whom treatment was discontinued after HBsAg levels were measured. A virological relapse was defined as an increase in serum HBV DNA level of >2000 IU/mL, and a clinical relapse was defined as a virological relapse with a biochemical flare, defined as an increase in the serum alanine aminotransferase level of >2 × upper limit of normal.
Results
After stopping ETV, virological relapse and clinical relapse were observed in 32 and 24 patients, respectively, during 20.8±19.9 months of follow-up. The cumulative incidence rates of virological relapse were 36.2% and 66.2%, respectively, at 6 and 12 months, and those of clinical relapse were 14.3% and 42.3%. The off-treatment HBsAg level was an independent factor associated with clinical relapse (hazard ratio, 2.251; 95% confidence interval, 1.076–4.706; P=0.031). When patients were grouped according to off-treatment HBsAg levels, clinical relapse did not occur in patients with an off-treatment HBsAg level of ≤2 log10 IU/mL (n=5), while the incidence rates of clinical relapse at 12 months after off-treatment were 28.4% and 55.7% in patients with off-treatment HBsAg levels of >2 and ≤3 log10 IU/mL (n=11) and >3 log10 IU/mL (n=28), respectively.
Conclusion
The off-treatment HBsAg level is closely related to clinical relapse after treatment cessation. A serum HBsAg level of <2 log10 IU/mL is an excellent predictor of a sustained off-treatment response in CHB patients who have received ETV for a sufficient duration.
INTRODUCTION
Chronic hepatitis B (CHB) is the leading cause of liver cirrhosis and hepatocellular carcinoma (HCC) worldwide, especially in Asia [1]. Since the replication of the hepatitis B virus (HBV) and the subsequent immune response are the main mechanisms by which chronic intrahepatic necroinflammation, progressive fibrosis, and liver cirrhosis develop, most practice guidelines recommend achieving long-term suppression of viral replication in CHB patients [2-4].
With the development of effective oral nucleos(t)ide analogues (NAs), prognosis of patients with CHB has improved significantly. NAs with a high potency and high genetic barrier, such as entecavir (ETV) or tenofovir disoproxil fumarate, effectively suppress HBV replication and hepatic necroinflammation, and they prevent disease progression and the development of complications [5-8]. However, the optimal duration of treatment is not clearly defined. The Western practice guidelines recommend long-term maintenance of NA treatment until the patient achieves hepatitis B surface antigen (HBsAg) seroclearance [2,9]. Considering the low incidence of HBsAg seroclearance following NA treatment [10-12] and the high relapse rate following the discontinuation of NA without HBsAg seroclearance [13-16], long-term treatment with NA is required in most CHB patients.
Recent studies have identified predictors of sustained off-treatment response following NA cessation, such as younger age [17,18], low alanine aminotransferase (ALT) [19] and HBV DNA [20] levels at baseline, and the duration of consolidation treatment [21-25]. Discrepant predictive values have been noted among various studies depending on the type of NA used, the criteria for the discontinuation of treatment, and the definition of relapse. In addition, the Asian Pacific Association for the Study of the Liver (APASL) recommends the discontinuation of NAs at 12 months of consolidation treatment after hepatitis B envelope antigen (HBeAg) seroconversion in HBeAg-positive patients, and at 12 months of consolidation treatment after achieving undetectable HBV DNA in HBeAg-negative patients [3]. However, CHB relapse occurs in most patients even though treatment is discontinued in accordance with this recommendation [23,24].
The serum HBsAg level correlates with the level of intrahepatic covalently closed circular DNA [26,27]. Furthermore, over the natural history of CHB, a low serum HBsAg level has been associated with improved immune-mediated viral clearance [28,29]. Therefore, serum HBsAg level could be a good predictor of sustained off-treatment response after NA cessation. Several studies have evaluated the efficacy of serum HBsAg level for predicting sustained responses following NA cessationbut the results have been conflicting so far. While some studies have validated serum HBsAg level as a predictor for sustained response [30-34], others have failed to demonstrate a relationship between serum HBsAg level and sustained off-treatment response [20,35,36]. Therefore, this study aimed to evaluate the efficacy of using the serum HBsAg level in predicting sustained off-treatment responses in ETV-treated CHB patients.
METHODS
Patients
Consecutive CHB patients who were treated with ETV for a sufficient duration and for whom treatment was thereafter discontinued after measuring serum HBsAg levels were included in this study. Patients with a previous history of treatment with NAs before ETV treatment or patients with HCC at the time of ETV initiation were excluded. CHB was defined as a positive serological test for serum HBsAg for at least 6 months. Virologic response was defined as a decrease in serum HBV DNA to undetectable levels by PCR assays [9]. Sufficient treatment duration was defined as ≥12 months of consolidation treatment after serum HBeAg clearance and virologic response in HBeAg-positive CHB patients and as ≥12 months of consolidation treatment after virologic response in HBeAg-negative CHB patients. Liver cirrhosis was diagnosed based on histology and/or imaging studies. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration and was approved by the Institutional Review Board (No: ED16137). A waiver of consent was obtained and the patient records were anonymized and de-identified prior to analysis.
Data collection
Clinical data including age, sex, daily alcohol consumption amount, and history of diabetes and hypertension were collected for each patient. Results of the laboratory tests (i.e., platelet count, international normalized ratio [INR], serum ALT, bilirubin, albumin, creatinine, and HBV DNA levels, and serum HBeAg/Ab) were collected at the time of ETV initiation. The duration of ETV treatment, the duration of consolidation treatment, the time of virological relapse, and the time of clinical relapse were also collected. The serum HBsAg level was measured at the end-of-treatment using the Architect HBsAg QT chemiluminescent microparticle immunoassay (Abbott Laboratories, Abbott Park, IL, USA). Laboratory tests, including serum HBV DNA and ALT levels, were performed for all patients every 3-6 months after the end-of-treatment. Patients were evaluated more often when clinically indicated. Virological relapse was defined as an increase in the serum HBV DNA level >2000 IU/mL, while clinical relapse was defined as a serum ALT level elevation >2 × upper limit of normal in addition to HBV DNA level >2000 IU/mL [20].
Statistics
Statistical analyses were performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean±standard deviation or number (%). The Mann-Whitney U test and chi-square test were used to compare continuous and categorical variables, respectively. The cumulative virological relapse rate was determined by the Kaplan-Meier method, and the difference between groups was determined by the log lank test. The Cox proportion hazard model was utilized to analyze factors associated with relapse. Significant factors (P<0.1) in the univariate analysis were subjected to multivariate analysis to determine independent predictive factors. All tests were two-tailed, and P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
Baseline characteristics are presented in Table 1. A total of 44 CHB patients were included in this study. The study population had a mean age of 44.6±11.4 years and 28 patients (63.6%) were men. Liver cirrhosis was diagnosed in 17 patients (38.6%). At the time of treatment initiation, serum HBeAg was positive in 25 patients (56.8%) and the mean serum HBV DNA level was 6.8±1.3 log10 IU/mL. Furthermore, the serum HBV DNA level was <7 log10 IU/mL in 22 patients (50.0%), while it was ≥7 log10 IU/mL in 22 patients (50.0%). The serum HBV DNA level was significantly higher in HBeAg-positive patients than in HBeAg-negative patients (7.2±1.0 vs. 6.2±1.5 log10 IU/mL, P=0.011), while other characteristics did not differ significantly between the two groups.
On-treatment virological response
Serum HBV DNA became undetectable in all enrolled patients and the mean time to undetectable HBV DNA was 5.4±0.4 months (median, 5.5 months). Serum HBV DNA became undetectable within 3 months, 3-6 months, and >6 months in 14 (31.8%), 24 (43.4%), and 6 (13.6%) patients, respectively. The time to undetectable HBV DNA did not differ between HBeAg-positive and HBeAg-negative CHB patients (P=0.222).
Serum HBeAg was cleared in all HBeAg-positive patients and the mean time to HBeAg clearance was 13.6±2.7 months (median, 9.8 months). HBeAg seroconversion was achieved in 7 of the 25 HBeAg-positive patients and the mean time to HBeAg seroconversion was 57.9±6.6 months.
Clinical course after the end-of-treatment
ETV was discontinued after 34.7±19.0 months of treatment (37.0±20.2 months and 31.6±17.4 months in HBeAg-positive and HBeAg-negative patients, respectively; P=0.356). ETV was continued for 29.3±18.6 months after undetectable HBV DNA in all enrolled patients (31.0±19.5 months and 27.1±17.7 months in HBeAg-positive and HBeAg-negative patients, respectively; P=0.50) and for 23.4±16.1 months after HBeAg clearance in HBeAg-positive patients. The duration of consolidation treatment was 25.0±16.7 months, which did not differ between the HBeAg-positive and HBeAg-negative patients (23.4±16.1 months vs. 27.1±17.7 months, P=0.475).
Serum HBsAg level at the end-of-treatment was 3.0±1.0 log10 IU/mL. Serum HBsAg levelswere ≤2, >2 ≤3, and > 3 log10 IU/mL in 5 (11.4%), 11 (25.0%), and 28 (63.6%) patients, respectively. The proportion of patients with an end-of-treatment HBsAg level of >3 log10 IU/mL was significantly higher in patients with positive HBeAg at baseline (20 of 25 patients, 80.0%) compared with those with negative HBeAg at baseline (8 of 19 patients, 42.1%; P=0.010). In addition, the proportion of patients with an end-of-treatment HBsAg level of >3 log10 IU/mL was significantly higher in patients with baseline HBV DNA >7 log10 IU/mL (19 of 22 patients, 86.4%) compared to those with baseline HBV DNA ≤7 log10 IU/mL (9 of 22 patients, 40.9%; P=0.004).
During a follow-up period of 20.8±19.9 months after the end-of-treatment, virological relapse was observed in 32 patients (20 and 12 patients whose baseline HBeAg was positive and negative, respectively). The cumulative virological relapse rates at 6, 12, and 24 months were 36.2%, 66.2%, and 77.3%, respectively (Fig. 1A). Clinical relapse was observed in 24 patients (16 HBeAg-positive patients and 8 HBeAg-negative patients). The cumulative clinical relapse rates at 6, 12, and 24 months were 14.3%, 42.3%, and 58.5%, respectively (Fig. 1B).
Clinical relapse occurred more frequently in the HBeAg-positive patients compared to the HBeAg-negative patients (P=0.049; Fig. 2A). The cumulative clinical relapse rates at 6, 12, and 24 months were 16.7%, 57.9%, and 73.7%, respectively, in the HBeAg-positive patients and 11.1%, 23.0%, and 37.7%, respectively, in the HBeAg-negative patients. The incidence of clinical relapse differed significantly according to the serum HBV DNA levels at the time of ETV initiation (P=0.038; Fig. 2B). The cumulative incidence of clinical relapse rates at 6, 12, and 24 months were 14.3%, 25.4%, and 37.4%, respectively, in patients with a baseline HBV DNA level <7 log10 IU/mL and 14.3%, 57.1%, and 78.6%, respectively, in patients with a baseline HBV DNA level ≥7 log10 IU/mL. Furthermore, the incidence of clinical relapse differed significantly according to the end-of-treatment serum HBsAg levels (P=0.010; Fig. 2C). Clinical relapse did not occur in patients with end-of-treatment HBsAg levels of ≤2 log10 IU/mL, while the incidence rates of clinical relapse at 6, 12, and 24 months after end-of-treatment were 18.2%, 28.4%, and 28.4%, respectively, in patients with end-of-treatment HBsAg levels of >2 and ≤ 3 log10 IU/mL and 15.4%, 55.7%, and 83.4%, respectively, in patients with end-of-treatment HBsAg levels of >3 log10 IU/mL.

Cumulative incidence of clinical relapse according to the baseline serum HBeAg level (A), baseline serum HBV DNA level (B), and end-of-treatment HBsAg level (C).
Of 24 patients with clinical relapse, ETV therapy was resumed after clinical relapse in all patients except one patient who was lost to follow-up after development of clinical relapse.
Predictive factors for clinical relapse
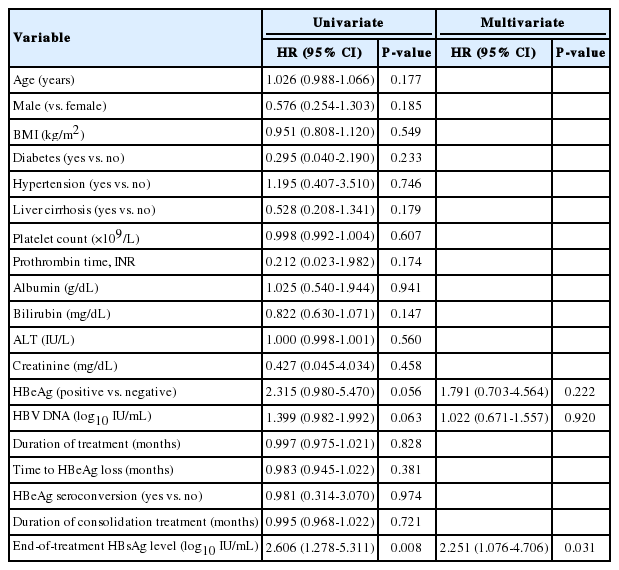
A univariate analysis revealed a trend of increased clinical relapse risk with positive serum HBeAg (P=0.056) and higher HBV DNA levels (P=0.063), while the serum HBsAg level was significantly associated with the development of clinical relapse (P=0.008; Table 2). Other variables including age, sex, and duration of consolidation treatment were not associated with the development of clinical relapse.
Multivariate analysis indicated that the serum HBsAg level was the only independent predictor of clinical relapse (β, 0.811; hazard ratio (HR], 2.251; 95% CI, 1.076-4.706; P=0.031), while HBeAg (β, 0.583; HR, 1.791; 95% CI, 0.703-4.564; P=0.222) and HBV DNA levels (β, 0.022; HR, 1.022; 95% CI, 0.671-1.557; P=0.920) were not significantly associated with the development of clinical relapse.
DISCUSSION
Although various studies have evaluated the predictive efficacy of the serum HBsAg level for a sustained off-treatment response, the efficacy is not clearly defined due to discrepant results. While some studies have reported that the serum HBsAg level is a good predictor for sustained response [30-32], other studies have not [20,35,36]. These discrepancies are not surprising since the type of NA used, duration of NA treatment, duration of consolidation treatment, and the definition of relapse differ across these studies [20,30-32,35,36]. We evaluated the predictive efficacy of the end-of-treatment HBsAg level in patients treated for a sufficient duration with ETV and report that the serum HBsAg level is an excellent predictor for sustained off-treatment response. Patients with end-of-treatment serum HBsAg levels of ≤2 log10 IU/mL showed a sustained response after discontinuation of ETV, which is consistent with previous studies [30-32]. Therefore, monitoring the serum HBsAg level may be useful for determining an optimal time for treatment discontinuation in patients who were treated with NAs for a sufficient duration.
There were some discrepancies between previous results and our results regarding significant predictors for sustained off-treatment response. Although the principle reasons for these discrepancies could be the different types of NAs used, different treatment discontinuation criteria, and the definition of relapse among the various studies, additional factors may be involved. First, previous studies have suggested that a younger age is a significant predictor for a sustained response [17,18]; but, we did not find any relationship between age and a sustained response. Although previous studies have shown a significantly lower relapse rate in patients less than 20 years [17] or 25 years of age [18], all enrolled patients in our study were 20 years or older, with only one patient being <25 years old. Furthermore, previous studies also reported that a lower baseline serum HBV DNA level is a significant predictor for sustained response [20,23,24], but the baseline HBV DNA level was not found to be associated with virological relapse in another study [31].
A previous study reported that the cut-off value of the serum HBV DNA level for predicting relapse as 2×105 IU/mL; however, we could not use this cut-off value as only five patients in our study group had a serum HBV DNA level <2×105 IU/mL. We instead used the median HBV DNA level (7 log10 IU/mL) as the cut-off value in our study. Even as the clinical relapse frequency was significantly higher in patients with baseline serum HBV DNA level of ≥7 log10 IU/mL, the baseline serum HBV DNA level was not significantly associated with the development of clinical relapse, as indicated by a multivariate analysis. This could be explained by the results of the end-of-treatment serum HBsAg level according to the baseline HBV DNA level.
Various previous studies have also suggested that a sufficient duration of consolidation treatment is required to achieve a sustained off-treatment response [21-25]. However, the duration of consolidation treatment was not related with a sustained response in this study. This could be due to the definition of sufficient duration of treatment in this study. All patients in this study were treated with ETV for more than 12 months after serum HBeAg clearance and virologic response in HBeAg-positive CHB patients and virologic response in HBeAg-negative patients. Previous studies have reported that prolonging the consolidation of treatment did not additionally improve the relapse rate [20,22,31].
There are discrepancies in the stopping rule used in this study and that of current practice guidelines [2-4]. First, ETV was discontinued in patients with ETV ≥12 months of consolidation treatment after serum HBeAg clearance and a virologic response in HBeAg-positive CHB patients in this study, while current practice guidelines recommend the discontinuation of NA treatment in patients who have achieved an undetectable serum HBV DNA level and, not HBeAg seroclearance, but HBeAg seroconversion in patients with a positive HBeAg at baseline [2-4]. However, HBeAg seroconversion is rare in patients with genotype C CHB compared to those with other genotypes [37]. In addition, in a previous Korean study, both HBeAg seroclearance and seroconversion were appropriate parameters for the discontinuation of NA [21]. Second, ETV was discontinued in patients with ≥12 months of consolidation treatment after virologic response in HBeAg-negative CHB patients in this study, while the APASL guideline recommends considering discontinuation of NAs in patients who have been treated for at least two years and have an undetectable level of HBV DNA [3]. However, this recommendation of duration of consolidation treatment was produced by the opinion of experts, and not by evidence [3]. Furthermore, the duration of the consolidation period was not associated with the development of clinical relapse in this study. Finally, 38.6% of patients included in this study had liver cirrhosis, while several practice guidelines recommend indefinite antiviral treatment in patients with liver cirrhosis because of the risk of hepatic decompensation or mortality after reactivation [2,4]. Although some patients in this study were combined with liver cirrhosis, their liver functions were absolutely normal at the time of end-of-treatment and there was no serious events in these patients. Similar to this study, 14-41% of patients with liver cirrhosis were included in previous studies [20,33,34].
There are several limitations in this study. First, the serum HBsAg level was not measured at the time of NA initiation as the test was not available. Analyses of baseline HBsAg level and its kinetics during treatment would have provided additional information, as a previous study has found a correlation between HBsAg kinetics and sustained off-treatment response in HBeAg-positive patients [30]. Second, this study was performed retrospectively, and although we enrolled all patients who met the inclusion criteria and tried to avoid a selection bias, it may not have not been sufficient. Finally, the sample size in our study was too small to confirm our results and large scale prospective studies are still needed to confirm the predictive efficacy of serum HBsAg level.
In conclusion, the serum HBsAg level at the end-of-treatment was closely related with off-treatment clinical relapse. Furthermore, a serum HBsAg level ≤2 log10 IU/mL is an excellent predictor for sustained off-treatment response in CHB patients who had received ETV for a sufficient duration.
Notes
Funding Support
This study was supported by the Research Supporting Program of the Korean Association for the Study of the Liver and by a grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Korea (HI10C2020).
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
APASL
the Asian Pacific Association for the Study of the Liver
BMI
body mass index
CHB
chronic hepatitis B
ETV
entecavir
HBeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
INR
international normalized ratio
NAs
nucleos(t)ide analogues