Autoimmune liver disease represented as primary biliary cholangitis after SARS-CoV-2 infection: A need for population-based cohort study
Article information
Dear Editor,
We read an interesting and informative guideline for liver disease management during the pandemic of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection by the Korean Association for the Study of the Liver (KASL), recently published in the Clinical and Molecular Hepatology [1]. The guideline also summarized the practical management in patients with underlying liver disease and SARS-CoV-2 infection. Meanwhile, there has been several articles discussing autoimmune hepatitis (AIH) after SARS-CoV-2 infection [2,3]. The possible mechanism for this phenomenon is that an increase in inflammatory cytokines, including tumor necrosis factor-α, interferon-γ and interleukin-10, after SARS-CoV-2 infection causes the onset of immune mediated diseases [4]. Moreover, it has been shown that SARS-CoV-2-specific CD8+ T-cells can cause AIH-like conditions after vaccination including our previous report [5,6], which may also occur after SARS-CoV-2 infection [3]. However, there has been limited data on the development of primary biliary cholangitis (PBC) after SARS-CoV-2 infection. Considering the suggested association between AIH and SARS-CoV-2 vaccination or infection, PBC can also be developed after SARS-CoV-2 infection as follows: a 56-year-old female patient with a 10-year history of hypertension and hepatitis C virus infection in a virologic cure state had normal liver function during follow-up even after third dose of SARS-CoV-2 Pfizer/BioNTech vaccination. Six months after the third dose of vaccination, she was infected with SARS-CoV-2 and referred to our hepatobiliary center for pruritus 3 weeks after infection. The patient had no history of herbal supplements, toxins, or alcohol consumption. Physical examination was normal, except for icteric sclera and jaundice. Liver function tests showed total bilirubin, 3.2 mg/dL; aspartate aminotransferase, 243 U/L; alanine aminotransferase (ALT), 630 U/L; alkaline phosphatase (ALP), 449 U/L; and γ-glutamyl transferase (GGT), 2765 U/L. All examined viral markers, such as hepatitis A, B, C, and E, Epstein-Barr virus, cytomegalovirus, and Herps simplex virus types 1 and 2, were negative. Autoantibodies including antinuclear antibody, anti-mitochondrial antibody, anti-smooth muscle, liver-kidney microsomal, and antineutrophil cytoplasmic antibodies were negative, with a normal level of immunoglobulin G (773 mg/dL). Dynamic computed tomography of the liver revealed normal findings. Percutaneous liver biopsy was performed to evaluate the possibility of autoimmune liver disease due to marked elevation of ALT, ALP, and GGT levels. Liver histopathology showed moderate portal inflammation with CD3+ T-cell dominant infiltration, rosette formation, and piecemeal necrosis. A considerable proportion of CD3+ T-cells were CD8+ T-cells, some of which targeted biliary epithelial cells. Granulomatous cholangitis and destruction and proliferation of the bile ducts without suppurative cholangitis were identified (Fig. 1A). After treatment with high-dose ursodeoxycholic acid (15 mg/kg), the liver enzyme levels returned to normal (Fig. 1B).
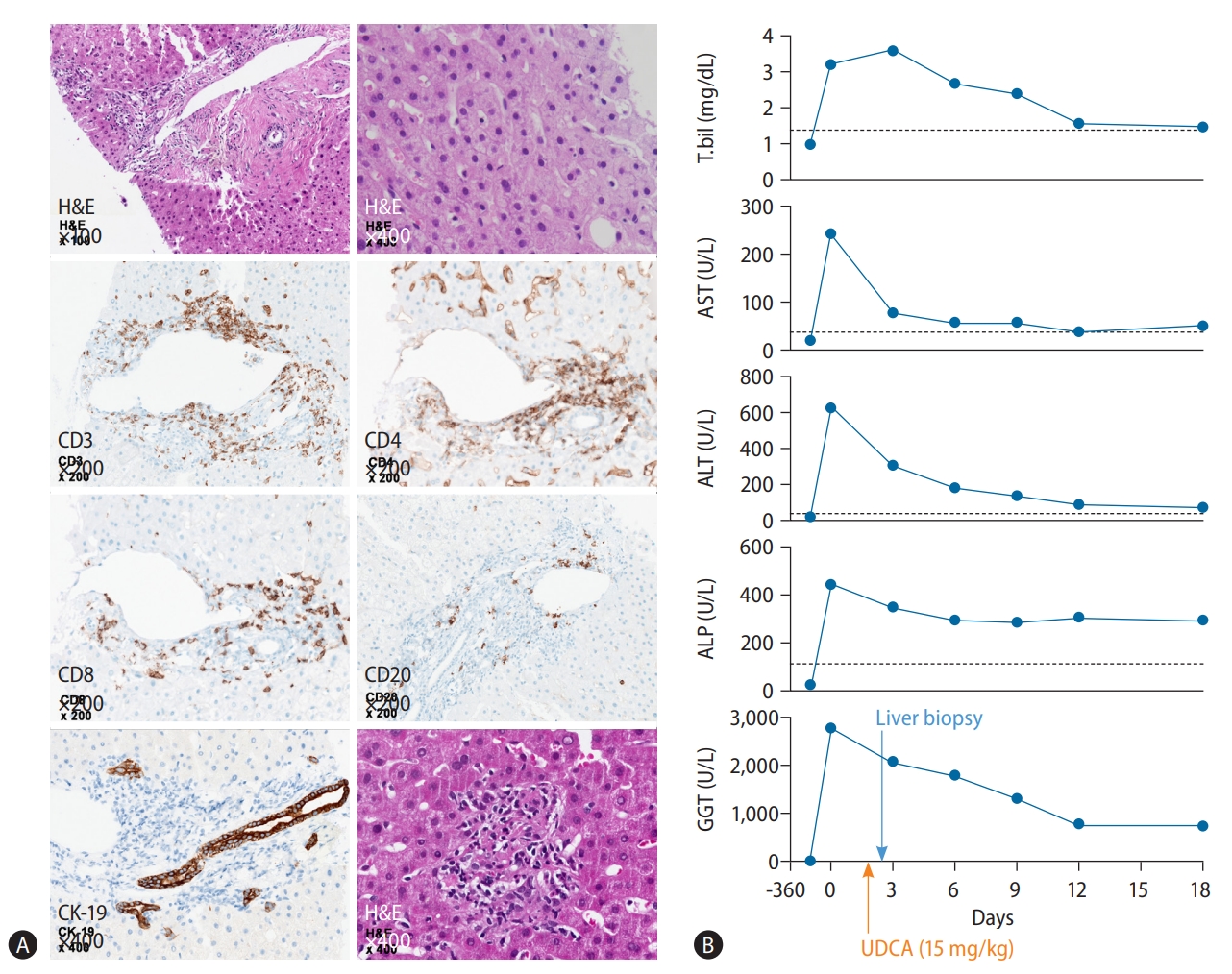
Histopathological findings and clinical course of the patient. (A) Moderate portal inflammation with piecemeal necrosis and rosette formation was noted on H&E staining. Infiltration of CD3+ T cells, which consisted mainly of CD8+ T-cells followed by CD4+ T-cells, and CD20+ cells, were identified in the portal and periportal areas. Some of the CD8+ T cells were targeting biliary epithelial cells. On H&E and CK-19 staining, granulomatous and nonsuppurative cholangitis with destruction and proliferation of the bile ducts was observed. (B) After treatment with high-dose UDCA (15 mg/kg), the patient’s laboratory results normalized in 2 weeks. H&E, Hematoxylin and Eosin; T.bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase; UDCA, ursodeoxycholic acid; CK, cytokeratin.
In our patient, the histological findings represented as nonsuppurative and granulomatous cholangitis with destruction and proliferation of the bile ducts were compatible with the findings of PBC [7]. Moreover, CD3+ T-cells, including CD8+ T-cells, were also dominant among the infiltrated cells in the portal area. As the interaction between autoreactive CD4+ and CD8+ T-cells and biliary pathways account for the development of PBC [7], virus-specific CD8+ T-cells might have played a crucial role in the development of PBC in our patient. Therefore, this letter supports the possibility of developing AIH-like conditions, including PBC, following SARS-CoV-2 vaccination or infection. As SARS-CoV-2 infection can increase liver enzymes and mortality in patients with cirrhosis, it has been recommended to monitor liver function and comorbidity in patients with SARS-CoV-2 infection [1,8,9]. Moreover, based on our report, it might be needed to consider the possibility of AIH or PBC in patients with elevated liver enzymes after SARS-CoV-2 infection. In conclusion, to verify the association of autoimmune liver disease and SARS-CoV-2 infection, further population-based cohort study is necessary to evaluate the incidence of AIH or PBC during the SARS-CoV-2 era.
Notes
Authors’ contributions
Soon Kyu Lee: Study concept and design, data acquisition and interpretation, drafting of the manuscript. Nara Yoon, Pil Soo Sung and Soon Woo Nam: Analysis and interpretation of data. Jung Hyun Kwon: Study concept, design and critical revision of the manuscript for important intellectual content.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (No. 2021R1I1A1A01050954; S.K.L.).
Abbreviations
AIH
autoimmune hepatitis
ALP
alkaline phosphatase
ALT
alanine aminotransferase
GGT
γ-glutamyl transferase
KASL
Korean Association for the Study of the Liver
PBC
primary biliary cholangitis
SARS-CoV-2
severe acute respiratory syndrome-coronavirus-2
