Serum transferrin as a liver fibrosis biomarker in patients with chronic hepatitis B
Article information
Abstract
Background/Aims
Transferrin and alpha-1 antitrypsin are reportedly associated with liver fibrosis. We evaluated the usefulness of serum transferrin and alpha-1 antitrypsin as new liver fibrosis markers in patients with chronic hepatitis B.
Methods
The study included 293 patients with chronic hepatitis B who underwent a liver biopsy between October 2005 and June 2009, and who had no history of hepatocellular carcinoma. Serum markers and liver fibrosis stages were compared.
Results
Univariate analysis revealed that age (P<0.001), serum platelet count (P<0.001), and serum alkaline phosphatase level (P=0.003) differed significantly between the patients with and without liver cirrhosis. Serum transferrin levels were significantly lower in advanced fibrosis than in mild fibrosis in both univariate analysis (P=0.002) and multivariate analysis (P=0.009). In addition, the serum transferrin level was significantly lower in cirrhotic patients than in noncirrhotic patients (P=0.020). However, the serum level of alpha-1 antitrypsin was not significantly associated with liver cirrhosis in patients with chronic hepatitis B.
Conclusions
Serum transferrin could be promising serum marker for predicting advanced liver fibrosis in patients with chronic hepatitis B.
INTRODUCTION
Liver fibrosis involves the excessive deposition of extracellular matrix components in the liver as a consequence of chronic liver damage.1 Accurate diagnosis of liver fibrosis is crucial to the management of patients with chronic hepatitis B (CHB) or chronic hepatitis C (CHC).2
Liver fibrosis is diagnosed by the histological analysis of liver biopsy specimens.3 However, liver biopsy is invasive and is limited by sampling errors, diagnostic inaccuracy, and hazards to the patient.4,5,6 Therefore, there is a strong demand for reliable, organ-specific, noninvasive biomarkers of liver fibrosis to replace invasive needle biopsy.4
Serum-based tests of liver fibrosis have attracted more attention in recent years. Serum markers offer several advantages: they are much less invasive, analysis can be automated, and test performance is reproducible. The most widely published serum marker panel of liver fibrosis is the Fibrotest, which includes α2-macroglobulin, apolipoprotein A1, haptoglobin, gamma glutamyl transpeptidase (GGT) and bilirubin.7,8,9,10,11 Most serum-based panels have been generated from studies of patients with chronic hepatitis C.7,12,13,14,15 On the contrary, in the case of CHB, similar models based on serum biochemical markers achieve a moderate correlation with liver fibrosis.16,17 Therefore, there would be a need for finding accurate and specialized markers in CHB patients for predicting the degree of liver fibrosis.
Many previous studies suggested excessive iron accumulation in the hepatocyte accelerate hepatocellular damage, liver fibrosis, and eventually increase risk of hepatocellular carcinoma.18,19 There were several studies to determine regulatory system of iron metabolism to maintain homeostasis and some studies proposed transferrin and hepcidin, which play a crucial role in iron metabolism, as surrogate markers for predicting severity of liver disease in the patients with chronic hepatitis C.20,21 Recently, Xu et al. performed proteomic study to identify reliable non-invasive serum biomarkers of liver fibrosis in patients with CHB, and they reported decreased level of serum transferrin in cirrhotic patients compared with non-cirrhotic patients.22 But there was no other study which validated serum transferrin as liver fibrosis marker in patients with CHB.
Alpha-1 antitrypsin (AAT) was known as most prominent protease inhibitor acting as anti-inflammatory cytokine and protecting the normal tissues during inflammation.23 AAT was highly expressed in sera of patients with severe CHB and hepatocellular carcinoma compared with control.24 There were many cytogenetic studies to find out the relationship of AAT and liver cirrhosis in patients with AAT deficiency.25,26 However, there were very limited studies to investigate the association between serum AAT and liver fibrosis in viral hepatitis.27
Our previous pilot experiment for evaluating serum biomarker of liver fibrosis in CHB patients revealed that serum transferrin and AAT were significantly different between patients with advanced liver fibrosis and patients with mild fibrosis. In this study, we tried to investigate and validate the role of serum transferrin and AAT for predicting liver fibrosis in relatively large cohort of the patients with CHB.
MATERIALS AND METHODS
Patient selection
Between October 2005 and June 2009, 293 consecutive patients with CHB who underwent liver biopsies in six medical centers (Ajou University Hospital, Hallym University Chuncheon, CHA University Hospital, Inje University Busan Paik Hospital, Pusan National University Hospital, and Catholic University St. Vincent's Hospital) in South Korea were recruited in this study. The inclusion criteria were CHB patients who underwent a liver biopsy with no history of hepatocellular carcinoma and who did not receive antiviral treatment for 6 months prior to commencement of this study. Patients with other causes of liver disease or decompensated cirrhosis were not included.
Informed consent to participate in the study was obtained from all study subjects. The study protocols were approved by the Institutional Review Board of Human Research of Ajou University Hospital and the local Research Ethics Committees at all participating hospitals.
Histologic examination
Liver biopsy specimens at least 10 mm long that contained at least 6 complete portal tracts were obtained from all patients. All biopsy specimens that met the inclusion criteria were staged by 2 central pathologists (YB Kim and YN Park). Hepatic fibrosis and necroinflammation were assessed using the Batts-Ludwig classification: F0=no fibrosis, F1=portal fibrosis without septa, F2=few septa, F3=many septa without cirrhosis, and F4=cirrhosis. Based on that system, grade 1 indicated necroinflammatory activities largely confined to the portal areas, grades 2-3 meant an extension beyond the portal areas, and grade 4 signified the bridging necrosis.
Biochemical analysis
Serum samples were obtained and routine blood tests were performed at the time of liver biopsy and processed immediately. The serum biochemical parameters included total bilirubin, alanine aminotransferase, aspartate aminotransferase, GGT, alkaline phosphatase (ALP), albumin, blood urea nitrogen, creatinine, prothrombin time (PT), blood glucose, triglycerides, and total cholesterol levels. Transferrin and AAT level were measured with the Cobas Integra immunoturbidimetric assay (Roche, Basle, Switzerland).
Statistics
The data about patients' characteristics are given as the mean±SD as appropriate. Comparisons between groups were performed by chi-square test, parametric independent t test, and 1-way analysis of variancetest, as appropriate; differences between means were considered statistically significant for P-value of < 0.05.Logistic regression analysis was performed for multivariate analysis. Receiver operating characteristic (ROC) curve was used to evaluate the diagnostic accuracy of serum transferrin for discriminating patients with advanced fibrosis (F3 or F4) from without advanced fibrosis (F0 to F2). And area under the curve (AUC) was calculated.All statistical tests were 2-sided and performed with SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
The baseline characteristics of the enrolled patients are depicted in Table 1. Among 293 patients, 205 patients (69.9%) were men; the mean age was 40.0 years (SD, 12.0 years; range, 14-70 years). Liver cirrhosis was present in 80 patients (27.3%). The distribution of the fibrosis stages was as follows: stage 0=12 patients (4.1%); stage 1=42 patients (14.3%); stage 2=82 patients (28.0%); stage 3=77 patients (26.3%); and stage 4=80 patients (27.3%). The grade of fibrosis was converted into a binomial variable of liver cirrhosis (F4, n=80) vs. no liver cirrhosis (F0, 1, 2, or 3, n=213) and mild fibrosis (F0, 1, 2, n=136) vs. advanced fibrosis (F3, F4, n=157).
Univariate analysis revealed that age (P<0.001), platelet counts (P<0.001), ALP (P=0.003) were significantly different between the patients with or without liver cirrhosis (Table 2).
Measurement of serum transferrin and AAT levels
We evaluated the correlation of serum transferrin and AAT concentration with liver fibrosis in the cohort of CHB patients.We performed a one-way analysis of variance to compare means of serum transferrin levels among the fibrosis stages. Serum transferrin levels were significantly different between the groups (P=0.017). Figure 1 shows the distribution of serum transferrin levels according to liver fibrosis stage. In the advanced fibrosis stage (F3 or F4), the serum transferrin level was significantly lower compared to patients with mild fibrosis stage (F1 or F2, P=0.001).
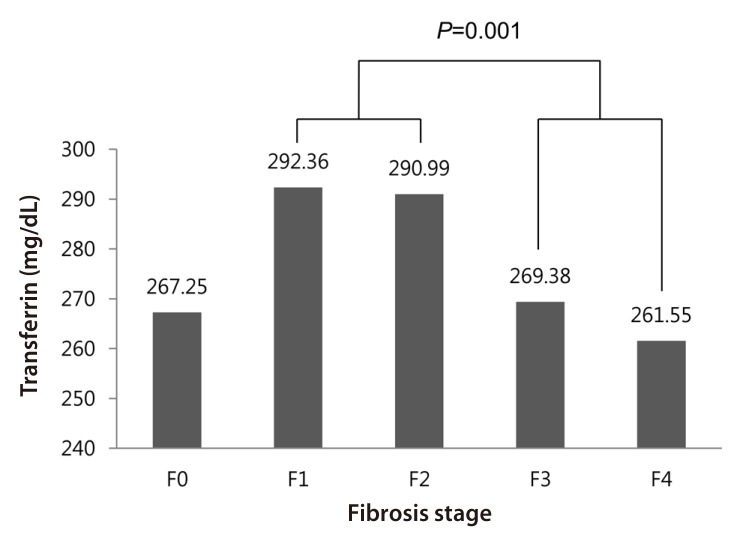
Distribution of serum transferrin levels according to liver fibrosis stage. The serum transferrin level was significantly lower in advanced fibrosis (stage F3 or F4) than in mild fibrosis (stage F1 or F2).
Comparing between patients with liver cirrhosis (F4) and without cirrhosis (F0 to F3), serum platelet count (P<0.001), serum ALP level (P=0.003), age (P<0.001) and serum transferrin level (P=0.020) showed significant differences in univariate analysis. Serum transferrin level was lower in cirrhotic patients when compared with non-cirrhotic patients. The variables of statistical significance (P<0.05) on univariate analysis were entered into the multivariate analysis. In the multivariate analysis, serum platelet count (P<0.001) and serum ALP level (P=0.003) were still statistically significant, but serum transferrin was not significant. (P=0.479). (Table 2)
Next, we analyzed the factors predicting advanced liver fibrosis. Serum transferrin level (P=0.002), platelet count (P<0.001), GGT (P=0.012), albumin (P=0.004), PT (INR) (P<0.001) and patients' age (P<0.001) showed significant differences in univariate analysis between patients with advanced liver fibrosis and without advanced fibrosis. In multivariate analysis, serum platelet count (P=0.001) and serum transferrin level (P=0.009) were revealed as independent factors for predicting advanced liver fibrosis. (Table 3)
Figure 2. shows the ROC curves of serum transferrin and serum platelet count to discriminate patients with advanced fibrosis from those without advanced fibrosis. The AUCs of serum transferrin and platelet count were 0.606 and 0.697, respectively (P=0.002, P=0.000).
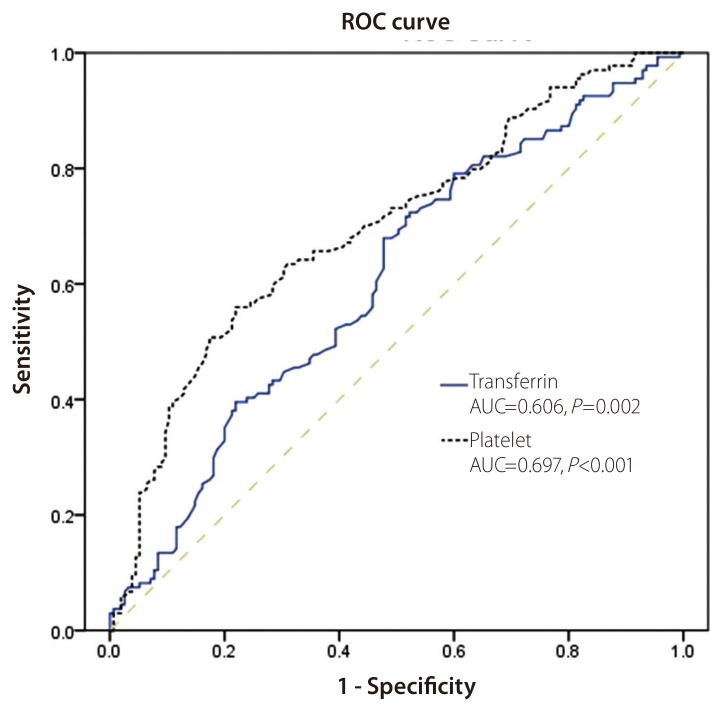
ROC curves for the serum transferrin level and the serum platelet count for discriminating chronic hepatitis B patients with advanced liver fibrosis from those with mild fibrosis. The area under the curve was 0.606 for the serum transferrin level (P=0.002) and 0.697 for the serum platelet count (P<0.001). ROC, receiver operating characteristics; AUC, area under the curve.
Serum AAT levels were not significantly different between patients with and without liver cirrhosis (Table 2). No significant differences were observed in serum AAT levels among the fibrosis stages (P=0.582).
Table 4 and Table 5. show results of univariate and multivariate analysis of transferrin as dependent variable and possible determinants, in order to verify transferrin as predicting marker of liver fibrosis. Serum ALP level (P=0.003), serum albumin level (P<0.001) and advanced liver fibrosis (P=0.011) were statistically significant in both univariate and multivariate analysis.
DISCUSSION
We performed the study to clarify the usefulness of serum transferrin and AAT levels in predicting liver fibrosis. Serum transferrin levels were decreased when mild fibrosis progressed to advanced fibrosis and cirrhosis, but serum AAT levels could not predict liver fibrosis in patients with CHB.
Hepatic iron deposition is well-known as leading cause of hepatic fibrosis and cirrhosis in hemochromatosis. Recently, hepatic iron deposition reported as important factor of hepatic fibrosis not only in the patients with hemochromatosis, but also in alcoholic liver disease, non-alcoholic steatohepatitis and chronic hepatitis C.28,29,30 Several investigators have suggested a relationship between the severity of chronic hepatitis and the degree of liver iron deposition.21,31
Transferrin plays a crucial role in iron metabolism. Transferrin, produced by the hepatocytes, is a glycoprotein consisted with a single polypeptide chain and two iron-binding sites.32,33,34 In our study, serum transferrin levels were elevated in the patients with mild fibrosis stage than those without hepatic fibrosis. However, it decreased when mild fibrosis progressed to advanced fibrosis and cirrhosis.Several reports on serum transferrin levels and liver fibrosis are comparable to ours. Potter et al. reported that transferrin level was increased in patients with alcoholic fatty livers but significantly decreased in cirrhotic patients.35 Ibrahim et al. reported that serum transferrin level was slightly increased in a pediatric liver disease group without liver cirrhosis, but was decreased in cirrhosis patients.36 These reports suggested that transferrin synthesis appeared to be raised during the early stage of liver disease, and thereafter it was decreased in advanced liver fibrosis, probably reflecting diminished synthetic capacity by the liver because of severe liver cell injury. Our study revealed that serum albumin level is also independent predictor of transferrin. This result suggests the possibility of an additional mechanism of transferrin suppression in advanced fibrosis other than decreased synthetic function. However, the exact pathophysiologic mechanism has not been clearly proven yet. Further basic research for identifying the mechanism will be needed.
There were several researches about iron metabolism alteration in chronic liver disease. Hepcidin plays a critical role in the maintenance of iron homeostasis. Several investigators have reported that hepcidin expression of hepatocyte was decreased at chronic hepatitis. Decreased hepcidin expression resulted in appearance of non-transferrin-bound iron, which known as a potentially toxic iron form, and to the development of parenchymal iron overload. Hepcidin expression is regulated by several factors including serum transferrin saturation.37,38 Considering results of our study, hepatic transferrin secretion might be increased to down regulate transferrin saturation due to compensate decreased hepcidin expression at mild fibrosis stage.
AAT is a protease inhibitor belonging to the serpin superfamily. It is an acute phase reactant, and its levels rise following tissue injury and inflammation.39,40 In our previous study, AAT level was significantly higher in patients with significant inflammation in univariate analysis but not in multivariate analysis.41 In current study, serum AAT was not associated with liver fibrosis in patients with CHB. Considering the function of AAT, it appeared to be more correlated with hepatic necroinflammatory activity than liver fibrosis. Further research will be needed to evaluate the clinical usefulness of AAT for predicting hepatic inflammation and fibrosis in patients with chronic hepatitis.
Our study had some limitations. First, our study did not separate HBeAg-positive patients from HBeAg-negative patients. The differences in clinical course or outcome in these two groups have been demonstrated in previous studies.16,42 Second, we didn't investigate other serum markers of iron metabolism like iron, ferritin, and haptoglobin which could affect the serum transferrin level. Third, our study did not clarify the relationship between serum transferrin level and hepatic iron deposition.
In conclusion, we showed that serum transferrin could be promising liver fibrosis marker in patients with CHB. Serum transferrin level was lower in the advanced fibrosis stage compared with mild fibrosis stage in the patients with CHB. Further study will be needed to establish the usefulness of serum transferrin level as potent liver fibrosis marker in patients with viral hepatitis. To our knowledge, this is the first validation study of serum transferrin level as a liver fibrosis-predicting marker in a relatively large number of CHB patients.
Acknowledgement
This work was supported by a grant from the Ministry of Health and Welfare, Republic of Korea (no. A102065)
Notes
The authors have no conflicts to disclose.
Abbreviations
AAT
alpha-1 antitrypsin
ALP
alkaline phosphatase
ALT
alanine aminotransferase
AST
aspartate aminotransferase
AUC
area under the curve
CHB
chronic hepatitis B
GGT
gamma glutamyl transpeptidase
HBeAg
hepatitis B e antigen
PT
prothrombin time
ROC
receiver operating characteristics
Std
standard