A case of focal nodular hyperplasia with growth progression during pregnancy
Article information
Abstract
Focal nodular hyperplasia (FNH) is the second most common benign solid tumor of the liver and is usually found in young females. In FNH, spontaneous bleeding or rupture rarely occurs and malignant transformation is unlikely. The etiology of FNH is unclear, but because of female predominance and young age at onset, it seems that female hormone has an important role for the development of FNH. Although the development and the complications of hepatocellular adenomas have been related to the use of oral contraceptives and pregnancy, the influence of oral contraceptives and pregnancy on the growth and complications of FNH is controversial. Most FNH are stable in size and rarely complicated during pregnancy. We describe here a case of FNH with growth progression during pregnancy in a 27-year-old female. Her course of pregnancy and delivery was uneventful. Two months after delivery, the size of FNH was decreased.
INTRODUCTION
Focal nodular hyperplasia of the liver is the second most common benign tumor, after cavernous hemangioma. This tumor is often diagnosed incidentally without symptoms, and the occurrence of complications is rare with no possibility of malignant transformation. Most of these tumors are known to have no change in size during pregnancy, and no complications such as hemorrhage and rupture. Therefore small-sized focal nodular hyperplasia without symptom diagnosed during pregnancy can be observed without treatment. If the tumor is large or symptomatic, the option of surgical removal could be considered, taking into account the possibilities of hemorrhage and rupture and the complications of surgery during pregnancy.
Here we report a case of focal nodular hyperplasia in a woman that enlarged during pregnancy and then decreased in size postpartum.
CASE
Patient
A 27-year-old married woman
Complaint
Further evaluation of an incidentally discovered hepatic mass.
Present illness
While being treated at another hospital for cough, vomiting and fever 1 month prior to referral, a 5 cm-sized mass was found in the right lobe of liver on abdominal ultrasonography. She was referred to our hospital for further evaluation of this hepatic mass.
Medical history
She had no medical history of diabetes, hypertension, hepatitis, tuberculosis, or trauma, and has been taking Synthyroxin 50 mg/day from 1 month ago due to hypothyroidism. She did not have any history of oral contraceptive use.
Social history
She had no history of alcohol intake or cigarette smoking.
Physical examination
Her blood pressure was 100/70 mmHg, body temperature was 36℃, pulse rate was 72 per minute, and respiration rate was 20 times per minute. On physical examination, her mental state was alert, and she showed no particular findings on head and neck, chest, and abdominal examination except that she was obese.
Laboratory findings
Complete blood cell count: WBC 4790/mm3, Hb 13.9 g/dL, and PLT 288,000/mm3. Serum biochemistry: total protein 8.1 g/dL, albumin 4.5 g/dL, AST 20 IU/L, ALT 23 IU/L, ALP 289 IU/L, total bilirubin 0.6 mg/dL, γ-GTP 58 U/L, BUN 10 mg/dL, and creatine 0.8 mg/dL. Prothrombin time was 11.4 sec., alpha-fetoprotein level was 2.6 ng/mL, HBsAg negative, HBsAb negative, and anti-HCV negative. On thyroid function test, TSH was 3.22 uIU/mL, and free T4 was 1.83 ng/dL.
Radiologic examination
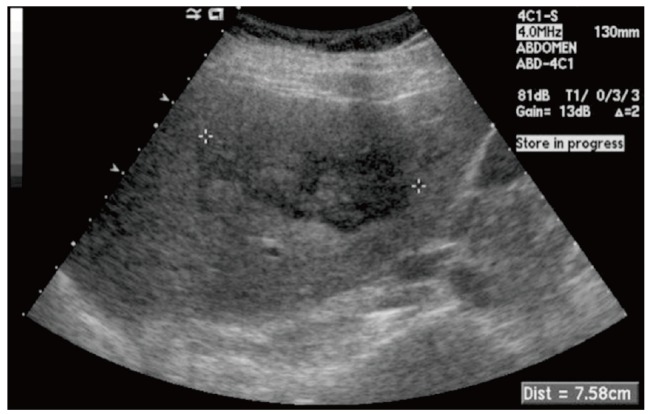
A 5 cm-sized hypoechoic mass was observed in segment 5/6 of the liver on abdominal ultrasonography. On abdominal computed tomography (CT), a well-enhanced 5 cm-sized mass was observed in segment 5/6 of the liver on arterial phase, and the lesion was isoattenuated on portal and delayed phases (Fig. 1). On magnetic resonance imaging (MRI), the mass showed low signal intensity on T1-weighted image, and weak high signal intensity on T2-weighted image. A strong contrast enhancement was seen on arterial phase using Gadobutrol (Gadovist®) as a contrast medium, and weak contrast enhancement was seen on portal and delayed phases (Fig. 2).

Contrast-enhanced abdominal CT shows a 5 cm-sized hepatic mass in S5/6. (A) Well-enhanced hepatic mass on arterial phase. (B) Isoattenuated hepatic mass on portal phase. (C) Isoattenuated hepatic mass on delayed phase.
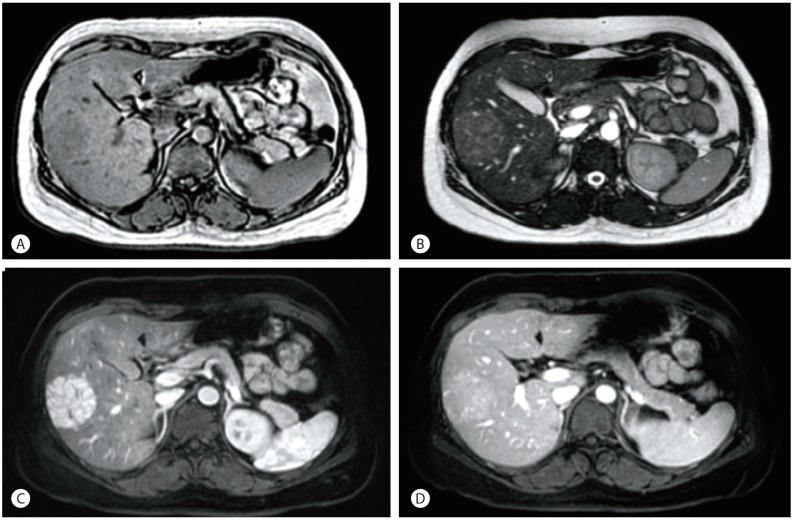
Abdominal MRI shows a 5 cm-sized hepatic mass in S5/6. (A) Hepatic mass shows low signal intensity on the T1 image. (B) Hepatic mass shows subtle high signal intensity on the T2 image. (C) Hepatic mass is well-enhanced on the arterial phase. (D) Hepatic mass is slightly enhanced on the portal phase.
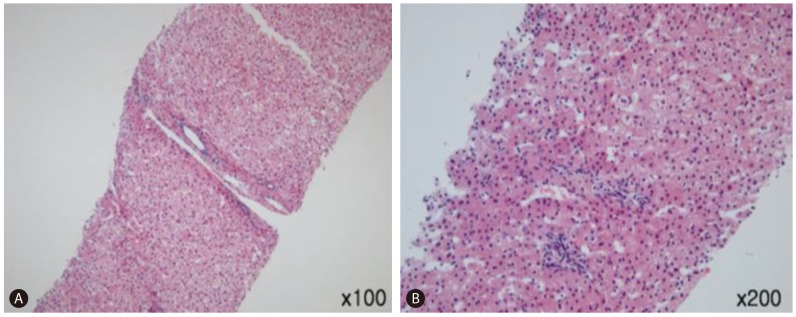
Histological findings
The findings obtained from percutaneous liver biopsy included increased number of liver cells, mild lobular inflammation, minimal steatosis, and focal portal triaditis. There was no evidence of hepatocellular atypia (Fig. 3).
Clinical progress
Although the percutaneous liver biopsy result was not diagnostic of focal nodular hyperplasia, the MRI findings were typical of focal nodular hyperplasia and therefore the patient was followed up in the outpatient clinic. The size of the mass was not changed after 3 and 6 months on abdominal CT. However, 1 year later when the patient was 7 months pregnant, abdominal ultrasonography demonstrated an increase in the size of the hepatic mass to 7 cm, and a serum alpha-fetoprotein level was 204.6 ng/mL. The size of the tumor increased further to 8 cm and 9 cm at her eighth and ninth month of gestation, respectively, on follow up abdominal ultrasonography (Fig. 4), and alpha-fetoprotein levels were further elevated to 308.0 ng/mL and 355.1 ng/mL, respectively. However, complications including hemorrhage, rupture, or necrosis of tumor were not observed. She did not have any particular symptoms. After an uneventful normal delivery without complications, a follow up ultrasonography was performed at 2 months postpartum and the size of the mass was not changed. The alpha-fetoprotein level was decreased to 2.4 ng/mL. At 5 months postpartum, the size of the hepatic mass was decreased from 9 cm to 7.5 cm (Fig. 5). The size remained the same for 1 year, and then it enlarged slightly to 8.0 cm. Up to now, there has been no change in size for 3 years, the serum alpha-fetoprotein level was within the normal range, and the patient is now being followed up in the outpatient clinic without particular symptoms.
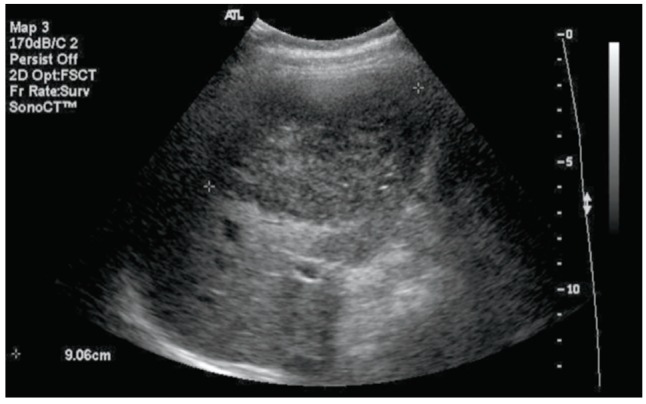
Abdominal ultrasonogram shows a 9 cm-sized hypoechoic hepatic mass in the right lobe at 34 weeks' gestation.
DISCUSSION
Focal nodular hyperplasia of the liver accounts for 2.5-8% of primary tumors occurring in the liver, and among benign tumors of the liver, it is the second most common one after cavernous hemangioma.1,2 It is found in all age groups in both males and females; however, it is more frequent in females, and more frequently between 20 and 50 years of age.3 Most patients with focal nodular hyperplasia do not have any symptoms. However, it is sometimes discovered because of abdominal discomfort or a palpable mass. Unlike hepatic adenoma which shows a high incidence in women of childbearing ages among other benign tumors occurring in liver, focal nodular hyperplasia does not show complications such as hemorrhage, and malignant transformation has not been reported. The etiology and pathogenesis have not been revealed yet, but based on the result of histopathological analyses, it is presumed to be caused by a hyperplastic response of the hepatic parenchyma against abnormal blood flow brought by malformed vessels at the center of the lesion.4
Hematological tests are usually normal, and liver function tests are also almost always normal, with normal range serum alpha fetoprotein levels. The alpha fetoprotein level was normal at diagnosis in this case, but it was increased up to 355.1 ng/mL during pregnancy. However, as the normal range for serum alpha fetoprotein is increased to 50-550 ng/mL during pregnancy5 and continued increasing pattern to late in pregnancy, it is possible that the elevated levels in our case was physiological.
For imaging diagnosis, abdominal ultrasonography, CT, and MRI can be used. A characteristic finding is the central stellate scar in the tumor. On ultrasonography, focal nodular hyperplasias appear as well-circumscribed nodules with variable degrees of echogenicity. On CT, they appear as tumors with low density or iso-density before injecting contrast medium, but after injecting contrast medium, contrast enhancement appears during the arterial phase in the tumor and not in the central scar portion. During portal and delayed phases, most of them appear as iso- or hypodense lesions. The central scars sometimes disappear when contrast medium is injected, and in some cases show delayed enhancement. MRI has a high diagnostic rate compared to abdominal ultrasonography or CT because of its sensitivity (68-70%) and specificity (98-100%) in the diagnosis of focal nodular hyperplasia.2,3,6,7 T1-weighted images show iso-signal or low signal intensity and T2-weighted images show weak high signal or iso-signal intensity. A strong contrast enhancement is shown in arterial phase after injecting contrast medium. The central scar appears as low signal intensity on T1-weighted image and high signal intensity on T2-weighted image. Sometimes, the central scar is not enhanced on arterial phase when contrast agent is injected, but it is enhanced in delayed phase. Recently, MRI using hepatobiliary-specific contrast agents such as gadoxetic acid and gadobenate dimeglumine is very useful in diagnosing focal nodular hyperplasia. In the hepatobiliary phase, focal nodular hyperplasia shows iso-density or high density.8,9 MRI using hepatobiliary-specific contrast agent is reported to have 96-97% sensitivity, 100% specificity, and 96% positive predictive value in diagnosing focal nodular hyperplasia.7,8 Existing extracellular fluid contrast agents circulate in the extracellular space after injection and are mostly excreted through the kidney. The differences in the blood flow between the mass and the liver determine the shape and other properties of the tumor. Because the hepatobiliary-specific contrast agents are taken up by hepatocytes and excreted in bile, the properties of the lesion can be shown depending on the functions of tumoral hepatocytes and bile excretion.8,9,10 Histologically, focal nodular hyperplasia consists of functional hepatocytes associated with abnormal blind-ending biliary ductules, which do not communicate with larger bile ducts. Hence, biliary excretion is slow compared with that of normal liver. Therefore, it shows contrast enhancement due to the retained contrast agent in the hepatobiliary phase.9
In this case, the image of hepatobiliary phase could not be obtained because MRI was taken using Gadobutrol(Gadovist®), an extracellular fluid contrast agent, before hepatobiliary-specific contrast agent was introduced to Korea, but a typical focal nodular hyperplasia was shown in the existing MRI with relatively high sensitivity and specificity. Also, it showed the findings adequate for focal nodular hyperplasia in ultrasonography and CT. Focal nodular hyperplasia can be diagnosed in 70-83% with a combination of radiological imaging tests.3,11,12
The usefulness of fine needle aspiration for a definitive diagnosis of focal nodular hyperplasia may be controversial.13 However, ultrasonography-guided liver needle biopsy is helpful for its diagnosis according to Fabre et al., and moderate subcapsular hematoma occurred in only 1 out of 30 patients, as a complication of liver needle biopsy.14
Even after careful diagnostic evaluation, patients with focal nodular hyperplasia undergo surgery due to uncertainty of diagnosis or risk factors of hepatocellular carcinoma. On gross examination, focal nodular hyperplasia appears as a mass that is well-demarcated from the adjacent normal liver, and the mass is generally non-encapsulated. The tumor consists of a number of nodules, and the scar located in the center of the nodule is surrounded by multiple radiating fibrous septa. Nguyen et al. divided focal nodular hyperplasia into the classical form that shows abnormal nodular architecture, malformed-appearing vessels, cholangiolar proliferation, and the nonclassical form which shows other characteristics.15 Although this case showed the findings of focal nodular hyperplasia on radiological imaging tests, the patient was married and planning to get pregnant. Therefore, to completely exclude the possibility of hepatic adenoma which can potentially result in complications such as hemorrhage and rupture during pregnancy and to render a definitive pathological diagnosis of focal nodular hyperplasia, percutaneous liver biopsy was performed. In this case, the typical findings of focal nodular hyperplasia were not observed. The pathological diagnosis of focal nodular hyperplasia may sometimes be difficult, for example, when the typical findings of the lesion are not present on the biopsied tissue or when the biopsy demonstrates atypical features of focal nodular hyperplasia.15 Recently, molecular biological methods using Angiopoietin1/Angiopoietin2 mRNA ratios, etc. have also been suggested for the differential between focal nodular hyperplasia and hepatocellular adenoma.11,16
Because focal nodular hyperplasia is often found in women in childbearing years, the relationships between female hormones such as estrogen or progesterone and occurrence and size increase of focal nodular hyperplasia are the subject of concern, but they are still controversial. In the past, it was thought that taking oral contraceptives is closely related to the occurrence of focal nodular hyperplasia, but recent reports show that the administration of oral contraceptives is not related to the occurrence of focal nodular hyperplasia, the size of lesions, and the number of them. Also, there was no change of the size during follow-up.17,18,19 In cases of focal nodular hyperplasia reported in Korea, there was no patient with history of oral contraceptives.
According to the medical eligibility criteria for contraceptive use announced by WHO in 2009, patients with focal nodular hyperplasia were classified as having more advantages than potential risks from taking oral contraceptives. Although there are few studies on the effect of pregnancy on the disease course of focal nodular hyperplasia, most of them did not show any changes during pregnancy, and the patients gave birth without particular complications.17,18,20 There are no reports in Korea regarding the natural course of focal nodular hyperplasia during pregnancy. According to foreign reports, Weimann et al.20 followed up 82 patients with focal nodular hyperplasia and reported that 10 patients among them got pregnant total 13 times with no change in the size of tumor. Mathieu et al.17 also followed up 216 patients with focal nodular hyperplasia, and reported that 12 patients among them were pregnant with no change of the size of tumor during their pregnancy. D'halluin et al.18 studied 44 patients with focal nodular hyperplasia in retrospective view. 6 patients were pregnant, 3 of them had no change in size of tumor during pregnancy, 2 patients showed decreased size, and only 1 patient showed 40% increased size of tumor. In these foreign reports, the diagnosis of focal nodular hyperplasia was mostly conducted by radiological imaging tests such as MRI, and if diagnosis was uncertain from the radiological imaging tests, the patients were diagnosed by the histopathological findings through percutaneous liver biopsy or surgical resection.
Among liver benign tumors which usually occur in women in childbearing years, unlike hepatic adenoma that may cause hemorrhage and rupture of tumor during pregnancy, focal nodular hyperplasia shows stable progress even in pregnancy and no complications. Therefore, focal nodular hyperplasias are observed without particular treatment.
In this case, the patient diagnosed with focal nodular hyperplasia on imaging tests showed an increased size of the tumor during pregnancy, but had an uneventful delivery without complications. There are no Korean reports of focal nodular hyperplasia in pregnant women so far. Because focal nodular hyperplasia is a benign tumor of liver that mostly occurs in women of childbearing age, it is necessary to have much concern and research for the progress during pregnancy of patients with focal nodular hyperplasia in the future.
Notes
The authors have no conflicts to disclose.
Abbreviations
CT
computed tomography
FNH
focal nodular hyperplasia
MRI
magnetic resonance imaging
WHO
World Health Organization