INTRODUCTION
Gilbert's syndrome (GS) is characterized by unconjugated hyperbilirubinemia that occurs in the absence of bilirubinuria, overt hemolysis, or liver disease.1 Decreased conjugation of bilirubin, due to diminished activity of the conjugation enzyme uridine diphosphate-glucuronosyltranferase (UGT1A1) is associated with an increased production of monoconjugated bilirubin.2 Hereditary spherocytosis (HS) is a common inherited hemolytic anemia; it is heterogeneous in its clinical presentation, inheritance, molecular basis, and biochemical phenotype.3 The characteristic features of HS include mild anemia, jaundice, and splenomegaly. The clinical severity varies, ranges from asymptomatic to severe life-threatening hemolytic anemia.3 GS concomitant with HS is associated with higher bilirubin levels and a 5-fold increase in the tendency to develop cholelithiasis.2 We describe moderate hyperbilirubinemia and gallstones in a male patient, who was given diagnosis of GS. Further evaluation of inappropriate hyperbilirubinemia led to detect a combination of HS with partial deficiency in ankyrin expression, and GS with heterozygous for a UGT1A1 allele with 3 mutations (T-3279G (UGT1A1 60), A (TA)7TAA (UGT1A1 28), and G211A (UGT1A1 6)). These combined disorders are the first report in a Korean. We also present the various strategies for making these diagnoses and review the literature on these concomitant disorders.
CASE REPORT
A 28-year-old man presented with watery diarrhea, abdominal discomfort, and fever. He had consumed a large amount of smoked, raw salmon the other day. He had been diagnosed with GS at the age of 12 years due to a history of intermittent unconjugated hyperbilirubinemia with normal liver function and underwent laparoscopic cholecystectomy for gallstones 1 year ago. The patient denied any family history. His height and weight were 179 cm and 69 kg, respectively. His body temperature was 37.6Ōäā. Clinical examination revealed icteric sclera, mild tenderness over the whole abdomen, and hyperactive bowel sound. Laboratory tests revealed the following findings; white blood cell count, 13,700/uL; hemoglobin, 11.0 g/dL; mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), 98 fL and 38 g/dL, respectively; aspartate aminotransferase, 21 IU/L; alanine aminotransferase, 20 IU/L; total bilirubin, 6.1 mg/dL; direct bilirubin, 0.8 mg/dL; gamma glutamyl transpeptidase, 11 IU/L; alkaline phosphatase, 54 IU/L. Abdominal computed tomography (CT) scan showed moderate splenomegaly and evidence of the previous cholecystectomy.
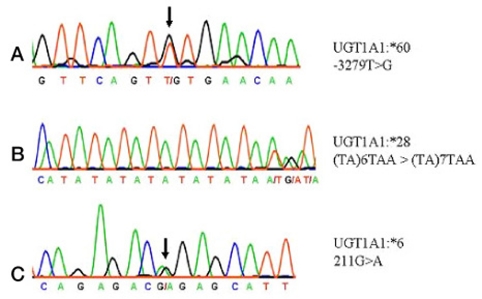
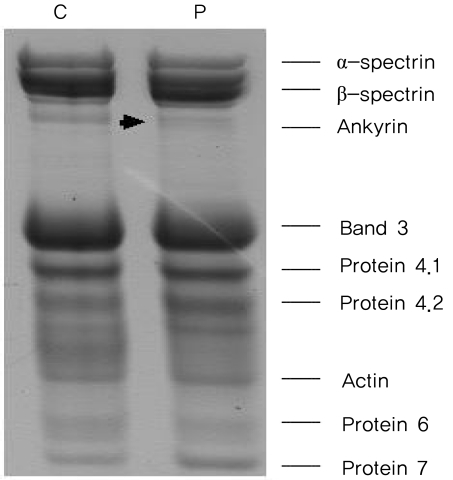
We analyzed the laboratory and the radiologic findings. The highest total bilirubin level recorded was 10.3 mg/dL (9.6 mg/dL, unconjugated). There were two conflicting findings for GS diagnosis: moderate splenomegaly and high values of unconjugated hyperbilirubinemia. Moreover, he had previously undergone surgery for gallstones. Therefore, we conducted further examinations to identify additional causes or other disorders. Extensive laboratory tests revealed the followings; corrective reticulocyte count, 6.3%; lactate dehydrogenase, 529 IU/; and haptoblobin, 2 mg/dL. The findings of Coombs' direct and indirect tests were normal. We detected many small, dense spherocytes in peripheral blood smear. The osmotic fragility was increased (begin: 0.52%, end: 0.44% of NaCl). We suspected GS coexisting with underlying chronic hemolysis. We could not recognize his hemolytic state because the lowest hemoglobin level recorded in the past laboratory findings was 13.3 mg/dL. At that time of cholecystectomy, the gallstones observed, i.e., a black pigment stone and a cholesterol stone, had a size of 7 and 6 mm, respectively. To clarify the diagnosis of GS and HS, we performed DNA sequencing of the UGT1A1 gene and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the red cell membrane proteins. Amplification of exons, the promoter region and enhancer regions (the phenobarbital responsive enhancer module, PBREM) of the UGT1A1 was performed by the polymerase chain reaction of genomic DNA.4 Amplified DNA fragments were sequenced directly.4 We investigated red cell membrane protein defects of the patient using SDS-PAGE.5 We found that the patient was heterozygous for a UGT1A1 allele with three mutations (T-3279G (UGT1A1 60), A (TA)7TAA (UGT1A1 28), and G211A (UGT1A1 6)) (Fig. 1). The 3-dimensional structure of the UGT1A1 protein was predicted by using the SWISS-MODEL (Fig. 2). By using the Fairbanks system, we detected the decreased expression of ankyrin on SDS-PAGE (Fig. 3). The patient was considered for splenectomy because of the likelihood of cholelithiasis despite the mild form of HS.
DISCUSSION
The UGT1 gene is located on chromosome 2q37. It encodes 9 functional proteins (UGT1A1, UGT1A3-10). The UGT1A locus includes 'first' exons and their associated promoters, which are spliced separately to four common exons (exons 2-5).6,7 Of the various UGTs characterized to date, particular attention has been paid to UGT1A1. Notably, this enzyme is solely responsible for bilirubin glucuronidation. Numerous mutations of UGT1A1 that influence bilirubin glucuronidation have been identified. Many of these have been linked to syndromes, particularly GS and the Crigler-Najjar syndromes type I and II.6 The incidence of GS is approximately 3~10% in the West and 3% in the East.8 GS arises usually due to polymorphism in a TA-repeat element, A (TA)5-8TAA, of the UGT1A1 promoter. Maruo et al.9 reported linkage of GS to A (TA)7TAA (UGT1A1 28) and T-3279G (UGT1A1 60). An in vitro expression study has revealed that the A (TA)7TAA transcription activity is one-third of the normal value, and that T-3279G reduces the transcriptional activity to 60% of the normal value.9 In addition, mutations in the coding region that result in reduced bilirubin-glucuronidation activity are also known to be associated with GS in an Asian population. The most prevalent mutation (13~23%) is G211A (UGT1A1 6), which results in the substitution of glycine for arginine at position 71 (G71R) of the UGT1A1 protein.6,10 An in vitro activity study showed that the G71R mutation reduced the total bilirubin glucuronidation activity by 50% as compared to its normal value.6
HS is a common inherited disorder that is characterized by anemia, jaundice, and splenomegaly. Although the clinical symptoms are variable, most patients presented with well-compensated hemolytic anemia. The primary lesion in HS is the loss of membrane surface area, leading to reduced deformability due to defects in the membrane proteins ankyrin, band 3, ╬▒ spectrin, ╬▓ spectrin, or protein 4.2.11 Spherocytic red cells, precociously trapped in the spleen, are phagocytosed by splenic macrophages which results in chronic hemolysis.12 Common complications are cholelithiasis, hemolytic episodes, and aplastic crisis. Removal of the spleen results in an increased red cell life span, decreased transfusion requirement, and a decreased incidence of gallstones. Splenectomy is thus the treatment of choice for moderate-to-severe forms of the disease.11,12
In our case, HS concealed by GS was revealed because the patient suffered from food poisoning. Further evaluation showed that the patient was heterozygous for triple different mutations in the UGT1A1 gene. In addition, the expression of the ankyrin protein was decreased in his erythrocyte membrane proteins. The unusually high levels of bilirubin observed in our case as compared to that reported for GS suggested the coexistence of an other disorder. However, the presence of two simultaneous causes of unconjugated hyperbilirubinemia may hamper the diagnosis. Sharma et al.13 reported a similar case of HS combined with GS.
GS is an heritable risk factor for gallstone formation resulting from impaired hepatic bilirubin clearance.14 Moreover, GS concomitant with HS has been associated with higher levels of bilirubin and a 5-fold increase in the tendency to develop cholelithiasis.2 Bile salts, calcium, or other biliary components could initiate the formation of pigment and cholesterol gallstones.2 Our case suffered from cholecystitis with a black pigment stone and a cholesterol stone and had cholecystectomy, however, the potential risk of cholelithiasis remained because of the chronic hemolysis. Therefore, we made a plan of splenectomy. However, it must be emphasized that splenectomy involves a surgical risk as well as the risk postsplenectomy sepsis and atherosclerotic events.10 It is not certain what extent the unconjugated hyperbilirubin level accounts for HS.
Two linked polymorphic mutations (A (TA)7TAA and T-3279G)9 and (A (TA)7TAA and G211A)10 of UGT1A1 were previously reported as the cause of GS. We performed amplification by polymerase chain reaction of genomic DNA and sequencing directly of promoter region, enhancer region exons of UGT1A1. To our knowledge, this is the first report of the concomitance of HS and 3 polymorphic, heterozygous mutations in the UGT1A1 gene. Thus our patient had three linked heterozygous polymorphism, A (TA)7TAA a TA insertion mutation in TATA box of the promoter region, T-3279G, located in the phenobarbital responsive enhancer module (PBREM), and G71R in exon 1. We present the 3-dimensional structure of the UGT1A1 protein that was predicted by using the SWISS-MODEL according to Li and Wu.15 It shows the substitution of glycine for arginine at position 71 (G71R) of the UGT1A1 protein.
We used SDS-PAGE for the analysis of erythrocyte membrane protein abnormalities. In addition, we also detected the partial deficiency of ankyrin. Lee et al.3 reported that ankyrin deficiency was the most common erythrocyte membrane protein defect in Korean HS patients.
We report an interesting case of HS with partial deficiency of ankyrin expression and GS with mutations in promoter, enhancer, and one exon of the UGT1A1 gene. Further evaluation is needed to determine the prognosis, and the therapeutic and genetic implications.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




