Prediction of compensated liver cirrhosis by ultrasonography and routine blood tests in patients with chronic viral hepatitis
Article information
Abstract
Background/Aims
Liver biopsy is a standard method for diagnosis of liver cirrhosis in patients with chronic hepatitis. Because liver biopsy is an invasive method, non-invasive methods have been used for diagnosis of compensated liver cirrhosis in patients with chronic hepatitis. The current study was designed to evaluate the usefulness of ultrasonography and routine blood tests for diagnosis of compensated liver cirrhosis in patients with chronic viral hepatitis.
Methods
Two hundred three patients with chronic viral hepatitis who underwent liver biopsy were included in this study and ultrasonography and routine blood tests were analyzed retrospectively. Ultrasonographic findings, including surface nodularity, parenchyma echogenecity, and spleen size, were evaluated. The diagnostic accuracy of ultrasonography and routine blood tests were examined.
Results
Discriminant analysis with forward stepwise selection of variables showed that liver surface nodularity, platelet count, and albumin level were independently associated with compensated liver cirrhosis (p<0.05). Cross-tabulation revealed that the following 4 variables had >95% specificity: platelet count <100,000 /uL; albumin level <3.5 g/dL; INR >1.3; and surface nodularity. If at least one of the four variables exists in a patient with chronic viral hepatitis, we can predict liver cirrhosis with 90% specificity and 61% sensitivity.
Conclusions
These results suggest that four variables (platelet count <100,000 /uL, albumin level <3.5 g/dL, INR >1.3, and surface nodularity) can be used for identification of liver cirrhosis in patients with chronic viral hepatitis with high specificity.
INTRODUCTION
Chronic hepatitis B (CHB) and C (CHC) are major causes of chronic hepatitis.1 Chronic carriers of the hepatitis B virus (HBV) are currently estimated to number of 350 million worldwide.2 Chronic carriers of hepatitis C virus (HCV) are estimated to number approximately 170 million people.3 Chronic hepatitis can progress to compensated liver cirrhosis. Compensated liver cirrhosis may then progress to liver failure and hepatocellular carcinoma with attendant complications, such as hepatic encephalopathy and varix bleeding. Therefore, early detection of liver cirrhosis in patients with chronic hepatitis has become an important clinical issue for physicians.4,5 Because the diagnosis of liver cirrhosis requires histologic demonstration of abnormal regenerative nodules surrounded by fibrosis, liver biopsy is still considered to be the gold standard for assessing fibrosis.6 Liver biopsy is limited, however, by the invasiveness of the procedure, cost, risk of complications (pain, bleeding, pneumothorax, bile peritonitis, and perforation), poor acceptance by patients, availability of expert practitioners, and intra- and inter-observer variability. An overall mortality one of 1 person per 10,000 has been reported in patients undergoing liver biopsy. False negative probability due to sampling error is reported to be 20~30%.6-9
It is necessary to establish non-invasive diagnostic methods to diagnose liver cirrhosis more easily. Non-invasive and more reproducible alternative methods, such as an elastography and blood indices, have been proposed and tested as a means for detection of liver cirrhosis, however, the clinical use is limited because those methods have not been confirmed and are difficult to use.10-12 A liver biopsy is rarely performed in the clinical diagnosis of compensated cirrhosis, and compensated cirrhosis is usually diagnosed by using blood tests and abdominal ultrasonography. However, the diagnostic criteria for liver cirrhosis and accuracy of the tests may vary according to the hospital. The present study was designed to determine the accuracy of abdominal ultrasonography and blood tests in the diagnosis of compensated cirrhosis in patients with CHB and CHC, and to determine the optimal point with high specificity in the diagnosis of liver cirrhosis.
MATERIALS AND METHODS
Patients
This retrospective study included 203 consecutive patients with CHB or CHC who underwent percutaneous liver biopsies, abdominal ultrasonography, and blood tests at Ajou University Hospital of Ajou University College of Medicine in Suwon, Korea between December 2003 and October 2009. Two hundred eighty patients underwent liver biopsies during the enrollment period. Seventy-seven patients were excluded because of poor quality ultrasonographic findings. Liver biopsies were performed to assess the degree of liver fibrosis and inflammation before antiviral treatment. Sonographic findings and blood tests were compared with histologic findings.
The patients included 167 males and 36 females (156 patients with CHB and 47 patients with CHC) with a mean age of 37.4 years (range, 18~72 years). Histologic examination revealed 167 F0-3 and 36 F4 (Table 1). In this study, CHB was defined as positivity for serum hepatitis B surface antigen, and a serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) level at least a 2-fold above the upper limit of normal for > 6 months. CHC was defined as positivity for serum anti-HCV antibody and HCV RNA. All of the patients were compensated for liver function. Exclusion criteria included autoimmune liver disease, evidence of alcoholic or fatty liver disease, and combined positivity for other hepatitis virus markers and other spleen and gallbladder-related diseases. Blood tests were performed within 1 week of abdominal ultrasonography and included a complete blood count, routine chemistries, such as albumin and bilirubin, and blood clotting factors, such as the international normalized ratio (INR).
Abdominal ultrasonography
Ultrasonography (US) examinations were performed within 1 week of the liver biopsy by two physicians (S. W. C. and J. Y. C.) using Logiq 7 (General Electric Co., Tokyo, Japan) or Prosound SSD-5000SV (Aloka Co., Wisconsin, USA) equipped with a 3.5~5.0 MHz convex probe. Ultrasonographic findings were reviewed by two gastroenterologists (S. W. C. and J. Y. C.) and a radiologist (J. K. K.). For 30 patients, the inter-observer variability of the US findings was tested to improve the objectivity of ultrasonographic findings. The reviewers were unaware of the pathologic diagnoses of the patients.
The US score was graded using three US variables which have been reported to be associated with the presence of cirrhosis and are currently utilized in clinical practice. Liver boundaries were carefully examined on the inferior surface of both lobes, especially in relation to the gallbladder and right kidney, and distinguished as normal (=0) and nodular (=1). Liver echogenecity was classified as normal (=0), coarse, and irregular (=1) in relation to the echogenecity and distribution of parenchyma echoes. Spleen size was assessed by the longitudinal size as this variable has been demonstrated to correlate with the actual spleen volume and is considered normal up to 12 cm. Each variable was classified as normal (=0) or frankly pathologic (=1). Therefore, a similar weight was given to the single variables, as there is no adverse evidence suggesting different relevance of one or more of these variables in the diagnosis of cirrhosis.
To analyze agreement between each examiner, kappa values were calculated. If there was inconsistency of the findings, representative values were defined when at least two inspectors came to the same conclusion.
Liver biopsy
Liver biopsies were performed using a 1.2-mm Menghini needle under informed consent. Tissue was more than at least 1 cm in length. Liver histology was evaluated semi-quantitatively according to the Metavir system. Fibrosis was classified into a 0~4 scale, as follows: F0, no fibrosis; F1, portal fibrosis; F2, periportal fibrosis; F3, septal fibrosis; and F4, cirrhosis based on the recommended criteria.13,14
Data analysis
SPSS 16.0 was used for the statistical data analysis. To assess the diagnostic accuracy of each non-invasive index, receiver-operating characteristic (ROC) curves were constructed and the areas under the ROC curves (AUROC) were calculated. Then, to evaluate the usefulness of the new predictive method, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. P values < 0.05 were considered statistically significant.
RESULTS
The interobserver agreement of ultrasonographic findings
Among the three reviewers, the kappa values for agreement of surface nodularity between 2 reviewers were 0.279 (P<0.001), 0.407 (P<0.001), and 0.335 (P<0.001). The kappa coefficients were between 0.2 and 0.4, which indicated fair agreement.
The kappa values for the agreement of liver parenchyma echo between 2 of 3 reviewers were 0.509 (P<0.001), 0.518 (P<0.001), and 0.435 (P<0.001). The kappa coefficients were between 0.4 and 0.6, which indicated moderate agreement.
Blood tests and ultrasonographic variables in the diagnosis of compensated liver cirrhosis
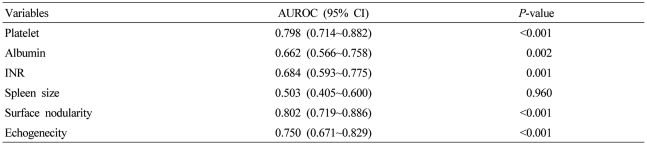
To validate the diagnostic values of non-invasive tests for cirrhosis, we calculated the AUROC of each variable (Table 2). The AUROC value of the spleen size for the diagnosis of cirrhosis was 0.503 and the p value of the test was 0.960. Thus, cut-off values could not be obtained. Five variables were identified as predictors of liver cirrhosis. These variables were as follows (in increasing order): albumin (P=0.002); INR (P=0.001); platelet count (P<0.001); surface nodularity (P<0.001); and parenchyma echogenicity (P<0.001). In particular, the AUROC value of surface nodularity, platelet count, and parenchyma echogenicity was high (>0.8).
Logistic regression analysis showed that surface nodularity was significantly associated with liver cirrhosis and the odds ratio was 12.645. A platelet count < 120,000/uL and an albumin level < 3.5 g/dL had significant odds ratio of 4.575 and 12.409, respectively. The other factors did not have a significant association with the diagnosis of liver cirrhosis (Table 3).
The usefulness of each variable in the diagnosis of compensated liver cirrhosis
To determine the diagnostic usefulness of each variable, cross-analysis was performed (Table 4). The sensitivity of each variable had a low range (5.56~41.67%), but the specificity was > 85%. A platelet count < 100,000/uL, an albumin level < 3.5 g/dL, a prothrombin time (INR) > 1.3, and surface nodularity had a specificity > 95%. With a platelet count cut-off value < 120,000/uL, sensitivity and specificity for the diagnosis of cirrhosis were 41% and 89%, respectively. Specificity increased to 96% and sensitivity decreased to 22% with a platelet count cut-off < 100,000/uL.

Diagnostic accuracy, sensitivity, and specificity of compensated liver cirrhosis by different variables
Cross-analysis was performed in combination with high specificity variables on the basis of the above results (Table 5). The combination of surface nodularity and parenchyma echogenecity satisfying two ultrasonographic variables showed a specificity of 97.6% and a sensitivity of 27.7%. Any 1 of 3 blood variables (platelet count < 100,000/uL, albumin level < 3.5 g/dL, and INR > 1.3) provided a sensitivity of 41% and a specificity of 93% for the detection of compensated cirrhosis. The combination of surface nodularity and one of the three laboratory variables provided 100% of specificity, but low sensitivity (range, 2~22%).
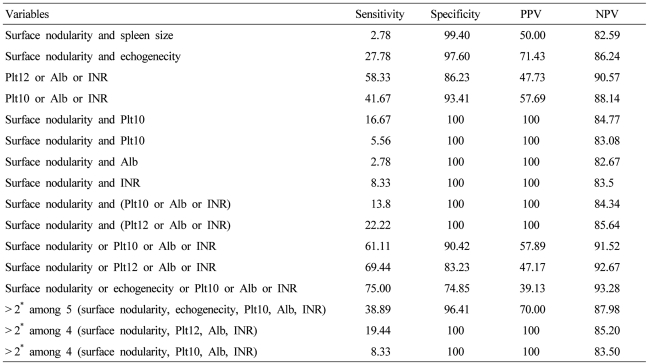
Diagnostic accuracy, sensitivity, and specificity of compensated liver cirrhosis by combination of variables
Based on these findings of at least one of the four variables (surface nodularity, platelet counts < 100,000/uL, albumin levels < 3.5 g/dL, INR > 1.3), cirrhosis was accurately identified with 90% specificity and 61% sensitivity. With a platelet cut off value of 120,000/uL, the sensitivity increased to 69% but specificity decreased to 83%. And the combinations satisfying two among many variables showed 40% or less of sensitivity.
DISCUSSION
Early diagnosis of liver cirrhosis in chronic hepatitis patients is critical because it can predict and reduce complications of cirrhosis and the occurrence of hepatocellular carcinoma. Guidelines for the diagnosis of compensated liver cirrhosis are rarely reported worldwide.15 In the absence of specific non-invasive diagnostic guidelines, clinicians use a variety of criteria. It is considered that these phenomena are caused by the limitations of non-invasive methods in the diagnosis of liver cirrhosis. Therefore, non-invasive diagnostic instructions of liver cirrhosis are very important to treat the patients and perform the research. For a non-invasive diagnosis of cirrhosis, ideal conditions require that the results are reliable, diagnostic tools are easy to perform, are inexpensive and commonly used, and deemed worthy by a number of checks.16 Methods satisfying these conditions include ultrasonographic imaging and blood tests. In the diagnosis of compensated liver cirrhosis, liver biopsy is the most accurate diagnostic method. However, a liver biopsy is performed in few patients in clinical practice. In most patients, the blood tests and abdominal ultrasonography are performed to diagnose compensated liver cirrhosis. The accuracy of these non-invasive diagnostic tests can vary depending on the physician.
In addition to ultrasonographic imaging, computed tomography and nuclear magnetic resonance imaging have been used to diagnose cirrhosis in some studies, but there was no distinct advantage over ultrasonography, and computed tomography and nuclear magnetic resonance imaging was not generally available.17
Because ultrasonography has been widely used for the observation of chronic hepatitis and hepatocellular carcinoma screening, the role of abdominal ultrasonography has been reported in the diagnosis of compensated cirrhosis. Gaiani et al.18 investigated the accuracy of an ultrasonographic score derived from liver, spleen, and portal vein features in predicting the final diagnosis in 212 patients with compensated chronic liver disease undergoing percutaneous liver biopsy. They identified liver surface nodularity and portal flow velocity as factors independently associated with cirrhosis (p<0.005), and a score based on these two variables correctly identified cirrhosis in 82.2% of cases (82.2% sensitivity and 79.9% specificity). Liver surface nodularity was associated with the diagnosis of cirrhosis in other studies.19-21 Enlargement of the caudate lobe had a high specificity, but low sensitivity (26~41%). Spleen size and echogenicity pattern also had a high specificity, but low sensitivity for diagnosing cirrhosis.20,21
In the current study, the ultrasonographic findings, such as liver surface nodularity, internal echogenicity pattern, and spleen size, were compared with pathologically-confirmed liver cirrhosis. In agreement with previous studies, liver surface nodularity had very high specificity (97%), but low sensitivity (33%). The sensitivity and specificity of the other two variables were lower than those of liver surface nodularity. The role of ultrasonographic examination in the diagnosis of early cirrhosis is limited due to low specificity, although sonographic variables could be combined. Therefore, ultrasonographic findings alone are insufficient as a screening test for liver cirrhosis.22
In addition to ultrasonography, there are many non-invasive methods using blood tests to diagnose liver cirrhosis. The serum fibrosis markers can be divided into direct and indirect markers. Direct markers are involved in the formation of extracellular matrix during fibrosis, and indirect markers reflect hepatic dysfunction. Several studies on the diagnosis of liver cirrhosis using direct fibrosis markers, such as collagen and hyaluronic acid, had a low sensitivity and specificity (approximately 80%), and fibrosis markers are expensive and not available in clinical practice.23-25 Indirect fibrosis markers include an indirect AST-to-ALT ratio (AAR) and an AST-to-platelet ratio index (APRI), Forn's index, and FIB-4. However, indirect fibrosis markers were not sufficient as a single indicator in diagnosing liver cirrhosis in many studies.11,26-28
Sebastian et al. published results showing that a fibroscan and blood tests can reduce the liver biopsy, but a fibroscan is an expensive piece of equipment. A fibroscan is not available for obese patients, and is not available for clinical practice.16
We evaluated the diagnostic accuracy of ultrasonography and routine blood tests in the diagnosis of compensated cirrhosis and established the diagnostic criteria. We investigated the diagnostic accuracy of the model consisting of four variables (surface nodularity, platelet count, albumin level, and INR). Based on the findings of at least one of the four variables, we identified cirrhosis accurately (90% specificity and 61% sensitivity). Variables used in this study included serum albumin and prothrombin time (INR) indicating the protein synthetic activity of liver and platelet count, reflecting portal hypertension. As these variables, except the platelet count, are used in the Child-Pugh classification, the specificity of the items appears to be high.
The albumin level and INR had > 95% specificity in identifying cirrhosis, but the sensitivity was very low. The platelet count had better sensitivity while maintaining high specificity compared to the albumin level and INR. A platelet count < 100,000/uL has been widely accepted as the cut-off level of portal hypertension.29 In this study, a platelet count < 100,000/uL had 22% sensitivity and 96% specificity in identifying compensated cirrhosis. When we defined a platelet count cut-off of <1,200,000/uL, the sensitivity increased to 41% with a minor reduction in specificity (89%).
This study has several strengths. First, liver cirrhosis can be predicted without special equipment in any hospital. Second, three experienced physicians reviewed ultrasonographic findings of 30 patients to objectify the subjective sonographic findings. Third, we suggested the standard criteria which are not unified in diagnosing liver cirrhosis on the basis of Korean data.
However, this study had several limitations. First, a retrospective study may have a bias in selecting patients and the number of patients was relatively small. Second, the study design was cross-sectional. The results of blood tests can vary depending on the patient condition. Thus, sequential measurements or mean values would be better as a representative value.
In conclusion, the model consisting of surface nodularity, platelet count < 100,000/uL, albumin level < 3.5 g/dL, and prothrombin time (INR) > 1.3 may be useful for the identification of compensated cirrhosis with a high degree of accuracy in daily practice.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (No. A050021).
Abbreviations
CHB
chronic hepatitis B
CHC
chronic hepatitis C
INR
international normalized ratio
ALT
alanine aminotransferase
AST
aspartate aminotransferase
AUROC
areas under the receiver-operating characteristic curves
PPV
positive predictive value
NPV
negative predictive value