Factors affecting drug-induced liver injury: antithyroid drugs as instances
Article information
Abstract
Methimazole and propylthiouracil have been used in the management of hyperthyroidism for more than half a century. However, hepatotoxicity is one of the most deleterious side effects associated with these medications. The mechanism(s) of hepatic injury induced by antithyroid agents is not fully recognized yet. Furthermore, there are no specific tools for predicting the occurrence of hepatotoxicity induced by these drugs. The purpose of this article is to give an overview on possible susceptibility factors in liver injury induced by antithyroid agents. Age, gender, metabolism characteristics, alcohol consumption, underlying diseases, immunologic mechanisms, and drug interactions are involved in enhancing antithyroid drugs-induced hepatic damage. An outline on the clinically used treatments for antithyroid drugs-induced hepatotoxicity and the potential therapeutic strategies found to be effective against this complication are also discussed.
INTRODUCTION
A large number of drugs have deleterious effects on liver and drug-induced liver injury (DILI) is a major clinical problem. Actually, drugs are responsible for a large number of cases of acute liver failure.1 Acetaminophen accounts for the approximately half of cases of DILI in the United States.2 In other world's regions, for instance in developing countries, other drugs such as antituberculosis medications might be the leading cause of DILI.3 In some cases of DILI such as acetaminophen-induced hepatotoxicity, the mechanism of liver injury is fairly well understood, hepatic damage is dose dependent, and the toxicity is reproducible in animal models. In contrast, a large number of other drugs have been associated with liver injury which their mechanisms of hepatotoxicity are less elucidated. Characteristically, these types of DILI are uncommon, not obviously related to drug dose, and multifactorial dependent; therefore, these reactions are termed 'idiosyncratic' DILI (IDILI).4 Many drugs have been identified to cause IDILI in humans.5 Antithyroid drugs-induced hepatic injury is categorized as IDILI.6
Due to its high morbidity and mortality, and unpredictable nature, DILI has become a major clinical challenge.7 Moreover, DILI accounts for a large number of drug withdrawals from the marketplace, or discontinuation of further development of a drug candidate. Hence, DILI not only cause patient hospitalization and sometimes need for liver transplantation and even death, but also involve the loss of potentially useful drugs.
Elucidating the mechanisms of toxicity and probable susceptibility factors which make patients vulnerable to DILI, will lead us to the safer pharmaceuticals development and reducing adverse drug reactions (ADRs).
Antithyroid drugs are chemically thioamide derivatives which are used more than 60 years for managing hyperthyroid patients. Methimazole and propylthiouracil (PTU) are two frequently used antithyroid agents in different countries. Despite the great effects of these drugs in controlling hyperthyroidism, their administration is associated with hepatotoxicity as a serious side effect.8,9 Hepatic injury induced by these agents could be so severe that might lead to hepatic failure and requirement for liver transplantation,10,11,12 and even death has been reported in some cases.13,14
Liver transaminase abnormality, that might indicate subclinical liver injury, is a common event after PTU administration.12,15 Kim et al. reported that asymptomatic elevation of serum ALT developed in 14.3% of investigated patients in a single center retrospective study.16 Yun et al. suggested that PTU therapy might be continued with caution in the presence of elevated liver transaminases, when no hyperbilirubinemia is present.15 However, regular monitoring of liver biochemistry has been suggested to allow discontinuation of PTU in suspected cases of hepatic injury.15,17 The diagnosis of PTU hepatotoxic effect should always be suspected in the patient receiving PTU therapy in whom clinical or biochemical evidence of acute hepatitis develops. The frequency of PTU-related severe liver damage is approximately 0.1% based on the available data.16,18 Nakamura et al. found a very high incidence of elevation of transaminase values with PTU in comparison with methimazole.19 Methimazole-induced hepatotoxicity usually develops in the first few weeks of drug consumption with an estimated incidence of 0.1-0.2%.6,20
The figure of hepatic injury induced by antithyroid agents seems different according the histopathological findings. The pattern of liver injury induced by methimazole is predominantly as cholestatic type,21,22,23 where those induced by PTU is of hepatocellular type in most cases.20,24,25 Pathologic findings of PTU-induced hepatic injury in human subjects revealed that this drug caused parenchymal necrosis with hemorrhage, collapse of lobular architecture, and preportal mixed inflammatory infiltrate.26 Hepatic abnormalities associated with methimazole are typical of a cholestatic process.22 Biopsy specimens from patients with methimazole-induced hepatic injury showed oedema and inflammation of portal tract with intracanalicular cholestasis and moderate microvascular steatosis.27,28
Fewer studies on the mechanisms and the pathogenesis of liver damage induced by these drugs are available. The mechanisms by which methimazole and/or PTU cause hepatotoxicity are not clarified completely yet, but it seems that these mechanisms might be different between currently available antithyroid drugs.29 Furthermore, the risk factors that render patients more susceptible to antithyroid drugs-induced hepatic injury are not fully recognized.
There are numerous factors that can contribute to differences among individuals in their sensitivity to xenobiotics. These include variation in age, gender, chemical metabolism in liver, underlying disease, coexposure to additional xenobiotics, and dietary status. Moreover, environmental and genetic factors have the capacity to apply significant influences on the onset of these determinants. The presence of susceptibility factors might make patients vulnerable to hepatic injury induced by xenobiotics (Fig. 1) and non-toxic chemical (e.g drugs) might become hepatotoxic in lower doses in the presence of risk factors (Fig. 1).
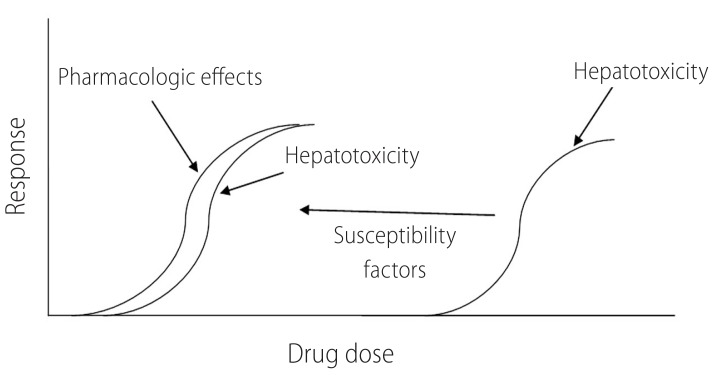
Susceptibility factors make patients more vulnerable to the toxicity induced by drugs. Presence of risk factors might make antithyroid drugs to be more hepatotoxic in lower doses.
The following is a review of data supporting different hypotheses on the factors which might influence antithyroid drugs-induced hepatic injury. In addition, an outline on the therapeutic strategies against antithyroid drugs-induced hepatotoxicity is given here.
Drug metabolism and immunological mechanisms
Drugs are generally transformed to biologically inactive structures and eliminated from the body, chiefly by hepatic metabolism. However, certain drugs undergo biotransformation to metabolites that can interfere with cellular functions. Drugs such as acetaminophen, tamoxifen,30 isoniazid,31,32 and amodiaquine33,34 are good examples of reactive, and possibly hepatotoxic, metabolite forming xenobiotics. The main theme of this section is to review the evidence for reactive intermediates as factors for antithyroid drugsinduced hepatotoxicity. Moreover, the role of variation in drug metabolizing enzymes between different individuals and its possible impact on the hepatic injury induced by these drugs is discussed here.
Methimazole is metabolized through cytochrome P450 (CYP) enzymes35,36 and flavoprotein mixed-function oxidase (FMO).37,38 Methimazole metabolism through CYP450 enzymes gives N-methylthiourea and glyoxal as two major metabolites.36 Nmethylthiourea is further oxidized with FMO enzyme to give sulfinic and sulfenic acid species.38 Glyoxal, is a reactive compound capable of interacting with different intracellular targets, such as proteins.39 Sulfenic acids are high electrophilic species that form irreversible adduct with cellular nucleophilic sites,40 and may have a role in methimazole-induced hepatotoxicity. N-methylthiourea is proposed to be the main methimazole reactive metabolite responsible for the hepatotoxicity induced by this drug.36 But, we recently showed that glyoxal also might has an important role in cellular injury induced by methimazole in an in vitro model of isolated rat hepatocytes.41
PTU is metabolized by glucuronidation in liver and prepared to exert from body (Fig. 2).42 In another study, the methylated and sulfate conjugate PTU was detected in rat urine.43 No PTU-related reactive metabolite(s) has been suggested yet to induce hepatotoxic reactions. But, it has been founded that myeloperoxidase (MPO)-mediated metabolism of PTU led to a reactive metabolite formation which covalently bounded to leukocytes proteins.44 This reactions might be responsible for agranulocytosis as a deleterious adverse effect of PTU.44,45

Antithyroid drugs glucuronidation. UGTs catalyze glucuronic acid transfer to drugs: a way to prepare these medications for excretion. (A) Proposed glucuronic acid conjugates of the methimazole. (B) PTU glucuronidation. The differences in UGT enzymes activities between different persons might alter antithyroid drugs pharmacokinetic which consequently lead to hepatotoxicity. UGT, uridinediphospho glucuronosyltransferase.
Many variations in drug metabolizing enzymes in humans has been identified.46 As methimazole is suspected to metabolized to reactive intermediate(s) which might involve in the hepatic injury induced by this drug,36,41 polymorphism in drug metabolizing enzymes might has a role in the idiosyncratic nature of the hepatic injury induced by this antithyroid medication. The exact CYP enzyme involved in methimazole and/or PTU metabolism is not characterized yet, hence further investigations are required to elucidate the possible role of enzyme variation in the hepatotoxicity induced by these drugs. Moreover, future investigations could focus on the possibility of reactive metabolite formation during PTU biotransformation in liver.
As mentioned, glucuronidation is a one of the metabolic pathways for antithyroid drugs (especially PTU) to preparing them for excretion (Fig. 2).42 The polymorphism in uridine diphosphoglucuronosyl transferase enzymes (UGTs) activity is also identified between human populations.47 Hence, another proposed mechanism for hepatotoxicity induced by antithyroid agents could be attributed to their impaired detoxification process, due to lower activity of UGTs. Consequently, some populations might be at a greater risk for these drugs to induce hepatic injury.
The selenium-containing analogues of antithyroid drugs are synthesized, and seems to have some advantages in comparison to their sulfur containing counterparts.48,49 These compounds could be newer antithyroid drug candidates. Since some investigations mentioned a potential role for "sulfur" atom in antithyroid agents to induce toxicity during metabolism,50 substituting "sulfur" with "selenium" might reduce the risk of antithyroid drugs-induced liver injury. However, the possibility if their serious adverse effects such as hepatotoxicity is lower than conventional drugs, remains to be elucidated in more future investigations.
Some investigations mentioned the critical role of cellular defense mechanisms such as glutathione (GSH) in preventing antithyroid drugsinduced hepatotoxicity.41,51,52 On the other hand, the liver GSH content might be variable in different situations. For example, it has been proven that liver GSH reservoirs are lower in malnourished patients53 and/or in some pathophysiological conditions such as alcoholism,54 so it seems that the risk of methimazole hepatotoxicity might be highest in these situations. Hence, particular attention should be given to the possibility that unexpected toxic reactions may be encountered under conditions of tissue GSH depletion. Physicians might advise their patient to avoiding alcohol consumption during antithyroid therapy.
Immunological mechanisms
The role of immune system in xenobiotics-induced hepatotoxicity is discussed in previous investigations.55 Immune-mediated reactions are suggested to play a role in antithyroid drug-induced hepatotoxicity.56 Although the mechanism(s) underlying this event is not fully elucidated yet 56, but cytokines are thought to play a role in immunemediated liver injury caused by methimazole.56 Cytokines could play an important role in DILI.57,58 Hence, another possible mechanism(s) for antithyroid drugsinduced hepatotoxicity could be immune system-mediated. The release of cytokines and autoantibodies are reported in antithyroid drugs-treated patients and/or laboratory animals.56,59 It has been shown that cytokine-mediated liver injury could has a critical role in methimazole-treated mice.56 Furthermore, some cases of PTU-induced hepatic damage are reported in which autoantibodies are demonstrated.60 Lymphocyte sensitization in a patient with neonatal liver injury probably by placental transfer of PTU has been reported.61 All these reports are in line with the hypothesis that patient immune system might play a role in the pathogenesis of hepatic injury induced by antithyroid medications. Antineutrophil cytoplasmic antibody (ANCA) positive cases are usually connected with deleterious immune system-mediated side effects such as vasculitis and autoimmune hepatitis induced by antithyroid medications.62,63 The presence of other autoantibodies such as preneuclear antineutrophil cytoplasmic antibody and antimyeloperoxidase antineutrophil cytoplasmic antibody all indicate that the immune system might be involved in antithyroid drugs side effects.64,65
"Hapten hypothesis" is an intriguing theory for immune-mediated drugs-induced hepatotoxicity.66 According to this hypothesis, reactive intermediates of different drugs, undergo covalent binding with different cellular proteins. The drug-protein complex is then recognized by immune system and consequently the activation of this system might led to hepatotoxicity.67 As mentioned, the reactive metabolites of antithyroid agents are capable of interacting with different targets including proteins. Hence, these modified proteins might act as haptens and consequently stimulate the immune system.
Recently an increasing interest has been made to new experimental models for studying idiosyncratic drug induced liver injury. "Drug-inflammation interaction" is one of these models.68 In this model, it is proposed that a modest inflammation will enhance some drugs induced hepatotoxicity.69 Evaluating antithyroid drugs-induced hepatotoxicity in such new experimental models, could be the subject of future investigations to elucidate the precise mechanism(s) of liver injury induced by these drugs.
It should be noted that PTU was widely used in the past in an attempt to treat alcohol-induced liver complications and severe alcoholic hepatitis.70,71 However, the beneficial effects of PTU against alcoholism appeared quite limited.70,71 Since alcoholism and alcohol-induced liver injury is an inflammatory-mediated process at least in part,72 one may argue that PTU might hasten liver injury in these patients. Although it seems that PTU doesn't increase liver-related events when it was investigated for treating alcoholic liver disease,71 but as many effective therapeutic options are developed against ethanol-induced liver injury,73 PTU administration against this complication seems to be just an antiquated and ineffective strategy. The interrelationship between inflammatory processes, PTU-induced hepatotoxicity and the druginflammationinteraction theory in alcoholic liver disease need more in depth experimental investigations.
Age
Age is a risk factor for drug-induced hepatotoxicity from specific medications.3 Younger age is a risk factor for particular medications such as valproic acid and aspirin.74 In contrast, older ages might be more susceptible to some other medications such as isoniazid,75 amoxicillin/clavulanate,76 erythromycin,3 and many other drugs.
Some comparative evaluation of adverse events including hepatotoxicity, related to antithyroid drugs has been done previously.29,77,78 In these investigations, the authors tried to find a relationship between the age of patients and the development of hepatotoxicity induced by methimazole and/or PTU. One of these investigations resulted that methimazole administration caused lower severe hepatotoxic events and vasculitis in children than the other drug, PTU.29 Interestingly, it has been reported that there are no reports of liver failure or liver transplantation in association with methimazole use in children in united states.11 Furthermore, there are fewer and less serious adverse events reported in FDA database for methimazole than for PTU.11 A substantial amount of data obtained from evidence based and prospective studies are indicated that methimazole is a safer pharmaceutical in management of hyperthyroidism in children.11,79 Some severe and even fatal PTU-induced liver injury cases are reported in children treated with this medication.80,81 Hence, some investigators suggested that PTU should no longer be used as a first line treatment for Graves' disease in children.11 Some studies showed that a shift in propylthiouracil prescription has occurred during years (Fig. 3) and methimazole is more prescribed in lower ages,78 probably due to its lower risk of liver damage in children.29,78
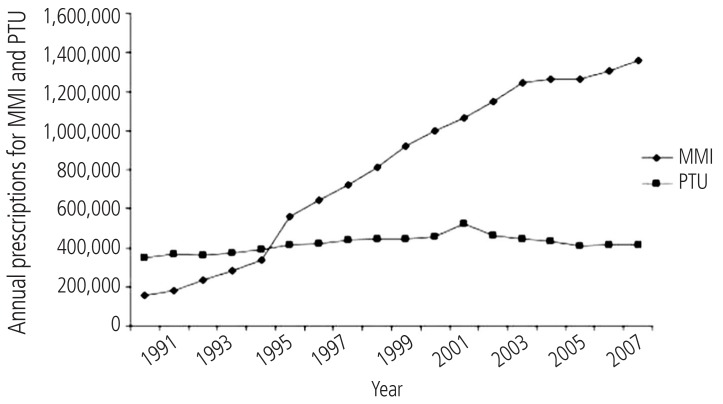
Antithyroid drugs prescription in US, from year 1991 to 2008. This figure is reprinted from reference "78" with permission from corresponding author ( Dr. David Cooper, Professor of Medicine, Division of Endocrinology, Diabetes, & Metabolism, The Johns Hopkins University, School of Medicine). MMI, Methimazole; PTU, propylthiouracil.
Although increased number of reports of severe propylthiouracil-induced hepatic injury in children favors methimazole administration (Fig. 3), but this doesn't mean that methimazole is completely safe in pediatrics.82 The mechanisms of hepatotoxicity induced by antithyroid drugs are not fully elucidated yet, and methimazole-induced liver injury categorized as an idiosyncratic reaction,6 hence physicians should be aware of this side effect in all age ranges including child's which in them might be a misconception about methimazole safety.
As the mechanism(s) of antithyroid drugs-induced hepatotoxicity is unknown completely yet, it is impossible to draw specific conclusions, but some probable hypothesis which differ pediatric and older patients in the sensitivity to antithyroid drugs is given below.
Besides the simple explanation that with increasing age the average consumption of drugs is higher (including multiple medications) and the risk for an additional disease that could influence the manifestation of idiosyncratic DILI is greater in older patients, there are other reasons based on a mechanistic base. For example, mitochondrial function (which is a frequent intracellular target of many drugs) is gradually weakening with advancing age, and cumulative oxidative damage to mitochondria keeps surging.83 Thus, oxidative damage to mitochondria is a critical factor of susceptibility. It seems that antithyroid drugs affect cellular mitochondria to induce cellular damage.41,84 On the other hand, significant differences in pharmacokinetic, and pharmacodynamics and enzymology are evident in pediatrics.85 These factors might bring this population different from older patients and antithyroid drugs hepatotoxic profile might be different in them. In addition, the variability in the activity of different enzymes which are responsible for the detoxification process of electrophilic species, namely glutathione transferase enzymes (GSTs), might also be involved in the different responses to antithyroid medications between adults and children.86 The critical role of glutathione in detoxification of antithyroid drugs is discussed in more details at next sections.
Although the choice of hyperthyroidism drug therapy might be a matter of clinicians' personal preference, but different factors such as patients' age should be noticed. Differences in the side effect profile of antithyroid drugs, might favor methimazole in children. On the other hand, methimazole should be administered cautiously in older persons with an appropriate dose, not only because of the concern for higher rate of agranulocytosis,87 but also probably because of more susceptibility of these patients to the hepatic injury induced by this drug.88
In conclusion, no clear and direct relationship between patients' age and antithyroid agents-induced hepatotoxicity has been founded yet, except for a higher rate of PTU-induced liver damage in pediatrics by an unknown mechanism(s).
Gender
Generally, drugs-induced hepatotoxicity is more prevalent in females.89 A large retrospective study has recognized that 76% of all patients who developed severe liver failure due to drugs were females.1 No clear reason has been identified for this increased susceptibility to many different drugs in women. Although more drugs are consumed by women as compared to men, and, although for some of the (dose-dependent) cases of DILI differences in the volume of distribution or drug-metabolizing enzyme expression have been suggested to account for the gender differences, there remains a significant part of all DILI cases where the sex-dependent increase in risk cannot be explained by increased exposure.90 Some authors has found no sex differences related to antithyroids-induced hepatotoxicity.82
Hyperthyroidism is more common in women91 and it has been found that the incidence of grave's disease is more frequent in females.92 Hence, any probable relationship between patients, sex and antithyroid drugs-induced hepatotoxicity might be simply due to the more rate of antithyroid drug prescription in females.
Drug interactions
Drug-induced hepatotoxicity can be affected by drug interactions. Generally, drug interactions occur when two drugs are co-administered and both are metabolized by the same CYP450. But, in another situation when a drug toxic metabolite is responsible for hepatotoxic reactions, coadministration of enzyme-inducing agents might deteriorate its hepatotoxic properties. Previously we showed that methimazole becomes severely hepatotoxic in enzyme-induced mice.52 This mentioned the importance of reactive metabolites in methimazoleinduced toxicity. Furthermore, other drugs with enzyme-inducing properties such as carbamazepine, isoniazid, etc. might interact with methimazole to lead hepatotoxic reactions. Hence, we might be able to propose that, the authorities should be cautious about coadministrating the drugs with hepatic enzymeinducing properties, such as phenobarbital, with antithyroid medications.
Underlying diseases
Grave's disease
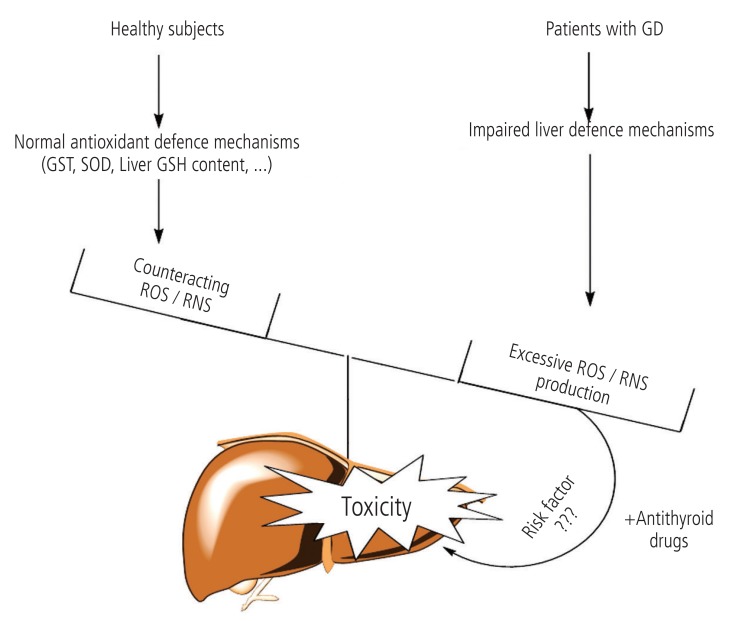
Several different disorders can cause hyperthyroidism. Graves' disease (GD), is the most common cause of hyperthyroidism in humans.93 Many investigations have been carried out on the complications which occur in hyperthyroid patients with GD. It has been shown that, the activity of different enzymes which are involved in the antioxidant defense mechanism of liver, are changed in hyperthyroidism.94 Komosinska et al. showed that glutathione reductase (GR) activity, was lower in hyperthyroid patients.95 In another investigation it has been observed that oxidative stress occurred in liver tissue of hyperthyroid animals probably due to impaired antioxidant defense mechanisms.96,97 As antithyroid drugsinduced hepatotoxicity is deteriorated in experimental models with defected liver protective mechanisms,41,51 the question arises if GD (the disease which antithyroid drugs are prescribed against) can sensitize individuals to the antithyroid medications' adverse hepatic effects (Fig. 4). The hypothesis that GD itself has a role in antithyroid drugs-induced hepatic injury, needs more controlled and in depth investigations to be clarified.
Therapeutic strategies against antithyroid drugs-induced hepatotoxicity
In many cases of antithyroid drugs-induced hepatic injury, no standard and specific treatment is available, except of drug discontinuation and monitoring patients' liver function during hospitalization.27,98 The most performed laboratory tests in antithyroid drugs-induced hepatotoxicity cases, are liver function tests as evaluated by serum aminotransferase levels (AST & ALT) and alkaline phosphatase (ALP) activity.8,99 Other assessments involve serum bilirubin, white blood cells count, prothrombin time (PT), and serum thyroid hormones and TSH levels.99,100 Moreover, abdominal ultrasonography is done to detect any cholestatic changes of patients' liver.6 Drug-induced hepatic injury could be certainly proven when other interacting factors such as viral hepatitis are ruled out.
Patients should be aware of symptoms which might indicate antithyroid drugs-induced hepatic injury. Indeed, all antithyroid taking patients must be trained to report symptoms such as abdominal discomfort and pain in upper right quadrant and other signs such as urine discoloration, which are connected to serious adverse reactions such as hepatotoxicity. As patients observed any of these symptoms, they must discontinue the medication and refer to their physician immediately. Liver biochemistry should be checked in clinically suspected cases.
As mentioned, no other medical intervention except for drug cessation and liver function monitoring has been made in the most cases of antithyroid drugs-induced hepatotoxicity.8,27,98 However, intra vascular glutathione was founded to be an effective treatment in a case of methimazole-induced hepatotoxicity and lowered transaminase levels in the patient.99 As mentioned, glutathione (GSH) has been proven to play a critical role in preventing methimazole-induced hepatic injury in different experimental models.41,51,52 Hence, using glutathione in patients with methimazole-induced liver injury seems to be a reasonable choice. In another study by Becker et al., it has been reported that corticosteroids might alleviate methimazoleinduced hepatitis.101 In another case, prednisolone administration rapidly recovered the patient from jaundice induced by antithyroid medications.102
Different protective agents have been identified to be effective against methimazole-induced hepatotoxicity in experimental models. For example we previously showed that organosulfur compounds103 and the amino acid, taurine,104 could ameliorate the toxic insult caused by methimazole. In another in vivo study, we showed that Nacetyl cysteine (NAC) successfully prevented methimazole-induced hepatic injury in different experimental conditions.52 These new protective strategies might help to develop a useful hepatoprotective agent against antithyroid drugs-induced liver injury in humans.
Future directions
Sixty years after antithyroid drugs introduction, these medications continue to be important in managing hyperthyroidism, but because of their potentially serious side effects such as hepatotoxicity, there is concern about their administration especially in pediatrics. This mentioned the need to consider the development of alternative antithyroid agents, in order to minimize the risk of hepatotoxicity and possible fatal outcome. Considering the development of newer antithyroid drugs with safer profile of toxicity is the need of hour. Some studies are conducted to synthesis newer analogues of currently available antithyroid agents.105,106,107 Taking more attention to the biological implications of these molecules, and testing their pharmacological actions in animal models, can lead to the development of newer and safer drugs for hyperthyroidism therapy. Moreover, elucidating the exact mechanism(s) of toxicity induced by antithyroid medications will lead us to better management of the developed hepatotoxic reactions.
Although methimazole has been reported to show a better overall safety profile than PTU, but considering probable susceptibility factors could give a better insight in its hepatotoxic profile and consequently reduce its adverse effects (Fig. 5). Some proposed risk factors which might make patients more vulnerable to methimazole-induced hepatotoxicity are summarized in Figure 5.
Recently, new models have been used to understand and predict drug-induced hepatotoxicity. Novel animal models such as lipopolysaccharide (LPS)-rodent model,108 and/or Sod +/- mouse model,109 are experimental tools to investigate about IDILI. The hepatotoxicity induced by antithyroid medications could be the subject of future studies in these novel experimental models, to improve our understanding of the clinical spectrum and mechanism of liver injury induced by these agents.
Conclusion Remarks
Clinical use of methimazole and PTU as convenient antithyroid medications are associated with the serious side effect of hepatotoxicity. Methimazole has been the most prescribed antithyroid drug these years (Fig. 3). Factors such as higher potency, convenient daily dosing, and the better toxic profile, might cause methimazole to be a more preferred drug in managing hyperthyroid patients (Fig. 3). But, physicians should be aware that methimazole can be associated with hepatotoxicity. Hence, considering susceptibility factors which might bring a person more vulnerable to antithyroid drugs-induced liver injury, might bright a better view on hyperthyroidism treatment and lowering the adverse drug reactions (Fig. 5). Patients who take antithyroid drugs should be advised for the risk of liver injury and be aware of possible susceptibility factors such as alcohol consumption, which might hasten liver damage induced by their antithyroid medications.
Acknowledgments
The authors thank Dr. David Cooper (Professor of Medicine, Division of Endocrinology, Diabetes, & Metabolism, The Johns Hopkins University, School of Medicine) for his kind permission to reprint Figure 3.
Notes
The authors have no conflicts to disclose.
Abbreviations
DILI
Drug-induced liver injury
GSH
Glutathione
MMI
Methimazole
PTU
Propylthiouracil
ROS
Reactive oxygen species
UGT
Uridinediphospho glucuronosyltransferase