Dobutamine stress echocardiography for evaluating cirrhotic cardiomyopathy in liver cirrhosis
Article information
Abstract
Background/Aims
The blunted ventricular systolic and diastolic contractile responses to physical and pharmacological stress in cirrhosis are termed cirrhotic cardiomyopathy (CCM). CCM has been known to involve multiple defects in the β-adrenergic signaling pathway. The aim of this study was to determine whether cirrhotic patients have blunted cardiac responses to catecholamine stimulation through dobutamine stress echocardiography (DSE).
Methods
Seventy-one cirrhotic patients with normal left ventricular (LV) chamber size and ejection fraction were enrolled. The LV systolic and diastolic functions were evaluated by two-dimensional and Doppler echocardiography at rest and during peak dobutamine infusion (40 µg/kg/min). An abnormal response was defined as a decrease of less than 10% in LV end-diastolic volume, a decrease of less than 20% in end-systolic volume, and an increase of less than 10% in LV ejection fraction (EF) at peak dobutamine infusion, based on previously used criteria. The early/late diastolic flow (E/A) ratio and diastolic parameters were also measured.
Results
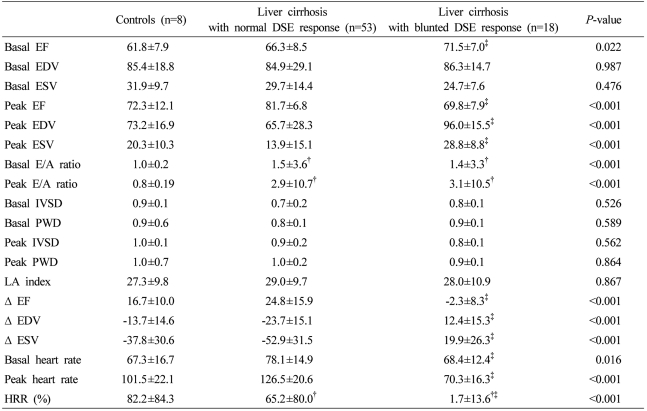
A blunted LV response to dobutamine was observed in 18 of 71 cirrhotic patients (25.4%). The baseline EF was significantly higher in 18 patients with a blunted DSE response than that of those with a normal DSE response (P<0.05). The baseline and peak E/A ratios, which are common diastolic dysfunction markers, were higher in the cirrhosis group than in the control group (P<0.001). No adverse events associated with DSE were observed.
Conclusions
Blunted cardiac responses to dobutamine stimulation, which are implicated in defects in the β-adrenergic signaling pathway, might contribute to the pathogenesis of CCM in patients with cirrhosis.
INTRODUCTION
Coronary artery disease which makes ischemic heart disease or cardiac dysfunction is rare in cirrhosis.1,2 However, most patients with cirrhosis have hemodynamic alterations characterized by increased cardiac output and decreased systemic vascular resistance and arterial pressure.3,4 Despite the increased resting cardiac output, the ventricular contractile response to stimuli is attenuated, a condition termed cirrhotic cardiomyopathy (CCM).5-7 In the absence of consensus definitions, the term 'cirrhotic cardiomyopathy' is defined at present as: 1) baseline increased cardiac output but blunted ventricular response to stimuli; 2) systolic and/or diastolic dysfunction; 3) absence of overt left ventricular failure at rest; 4) electrophysiological abnormalities including prolonged Q-T interval on electrocardiography and chronotropic incompetence, and not all features are required for the diagnosis. Other causes leading blunted ventricular contractile response to stimuli should be ruled out before defining CCM. CCM is generally latent but unmasked when the patient is subjected to stress in the form of infection, exercise, drugs, hemorrhage, and surgery, such as liver transplantation or insertion of transjugular intrahepatic portosystemic stent-shunts (TIPS). Clinical features include structural, histological, electrophysiological, systolic, and diastolic functional abnormalities. Although CCM is clinically important, the screening methods and the prevalence of CCM are not clear yet. Many factors are suggested as a mechanism of CCM and impaired β-adrenergic receptor signal transduction is one of them.8,9 Hence, we assumed that ventricular systolic and diastolic function in a CCM patient would be blunted by norepinephrine or other catecholamines stimulation such as dobutamine (Fig. 1). Therefore, in this study, we aimed to evaluate whether cardiac responses to stimulation by catecholamine are blunted in patients with cirrhosis through dobutamine stress echocardiography (DSE).

Mechanism of dobutamine stress echocardiography (DSE) as a screening test for cardiac dysfunction in liver cirrhosis, such as cirrhotic cardiomyopathy (CCM). Heart-cell contractility is determined mainly by the stimulatory β-adrenergic receptor system. When stimulated by norepinephrine or other catecholamines, the β-adrenergic receptor-ligand complex couples with the G-protein to stimulate membrane-bound adenylate cyclase, generating cAMP, which phosphorylates several proteins, leading to intracellular calcium fluxes. The calcium then causes actin-myosin cross-linking, and thus cellular contraction. In CCM, the β-adrenergic receptor signaling pathway shows defects at multiple points, so dobutamine, an adrenergic stimulant, could not induce a myocardial response.
βAR, β-adrenergic receptor; AC, adenylate cyclase; NO, nitric oxide; CO, carbon monoxide; iCa, intracellular free calcium-denotes an inhibitory stimulatory influence.
MERTERIALS AND METHODS
Patients
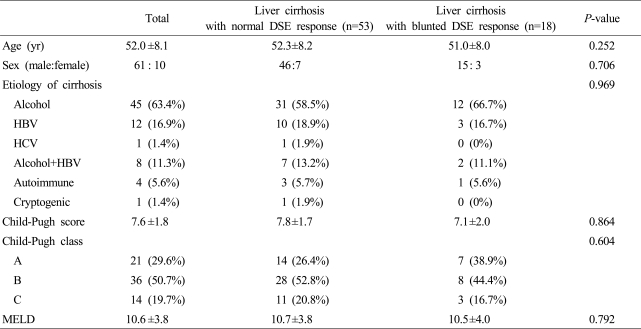
Seventy-one patients (60 males, 11 females) with liver cirrhosis were recruited from the Wonju College of Medicine Wonju Christian Hospital between May 2006 and June 2008. Cirrhosis was established by biopsy proven in 47 patients, and by presence of varices, laboratory data and image studies including ultrasonography and computer tomography scan in remaining patients. Cirrhosis was caused by alcohol in 45 patients, by hepatitis B virus in 12, by hepatitis C virus in one, by a combination of alcohol and hepatitis B virus in eight, by autoimmune hepatitis in four, and by cryptogenic in one patient. Twenty-one of these patients belonged to Child-Pugh class A, 36 were class B, and 14 were class C. The patients' characteristics are summarized in Table 1. All patients had abstained from alcohol for 6 months before the study. An accurate history was recorded, and a complete clinical examination including chest radiography, electrocardiography, pulmonary function tests, and routine laboratory screening tests were carried out at the time of inclusion. No patient had major cardiovascular or lung disease, renal disease, recent gartrointestinal bleeding episodes (<15 days), severe anemia (Hb<10 g/dL), or hepatocellular carcinoma. Especially, patients who showed abnormal electrocardiography or regional wall motion abnormality, valvular heart disease in echocardiography were excluded at baseline enrollment. No patient took any drug that had an effect on hemodynamics until the study was completed. Eight age-and sex-matched healthy, non-smoking individuals with no history of cardiac or liver disease served as controls. They had normal results from physical examinations, liver chemistry tests, complete blood count, chest radiography, and electrocardiography. The ethics committee of Wonju College of Medicine Wonju Christian Hospital approved all components of our study and patients and control subjects provided written informed consent.
Dobutamine stress echocardiography (DSE)
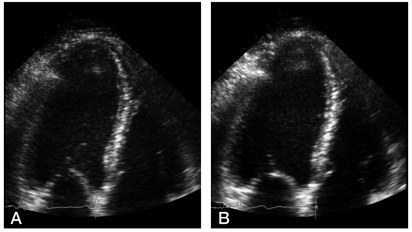
Patients and controls were studied the morning after an overnight fasting. After a 30-min period in the supine position, heart rate and arterial pressure were recorded and then DSE was taken. In resting conditions, a two-dimensional echocardiographic examination of the left ventricle was performed using a wide-angle phased-array imaging system (General Electric, Vivid 7 Dimension) equipped with a 4-MHz transducer and recorded on EchoPAC (General Electric). A 12-lead ECG was also recorded with the patient at rest. Dobutamine was administered intravenously with an infusion pump from 5 µg/kg/min to a peak infusion rate (40 µg/kg/min) and continued for 5 min. DSE was continuously monitored and recorded at rest and during dobutamine infusion (Fig. 2, 3).10 With echocardiography, cardiac structural and functional indices such as ejection fraction (EF), end-diastolic volume (EDV), end-systolic volume (ESV), early/late diastolic flow ratio (E/A ratio), interventricular septal diastolic wall thickness (IVSD), left ventricular posterior wall diastolic thickness (PWD) at baseline and peak infusion, and their percent changes were estimated. Off-line analysis of echocardiographic images was performed by two investigators under blinded conditions. Agreement between the two observers was required for test results to be interpreted as positive. In the case of disagreement, the judgment of a third observer was conclusive.

Normal left ventricular response to DSE in liver cirrhosis. (A) Apical four-chamber view at baseline. (B) At peak dobutamine infusion (40 µg/kg/min), the left ventricle shows a normal contraction response.
Definition
An abnormal volume and left ventricular EF response is defined as a blunted DSE response with a less than 10% decrease in end-diastolic volume, a less than 20% decrease in end-systolic volume, and a less than 10% increase in EF at peak dobutamine infusion.10 We also measured chronotropic response by heart rate (HR) reserve (HRR)={[(peak HR-HR at rest)/(220-age-HR at rest)]×100} and a chronotropic incompetence was defined as HRR less than 80%.11,12
Variability analysis
Intra-observer variability, in regard to calculating left ventricular volume, was assessed by the investigator in the first 30 dobutamine stress tests of this study. At baseline, the mean percent variability values in two different blind evaluations performed 30 days apart for end-diastolic and end-systolic volumes were, respectively, 6±4% and 6±5%. At peak stress, the mean percent values were, respectively, 7±5% and 7±6%.
Statistical analysis
Data were expressed as mean ± SD unless otherwise indicated. Comparison of groups (cirrhotic patients with blunted DSE response, normal DSE response, and controls) were performed by the Mann-Whitney U test and the Kruskal-Wallis test as non-parametric analysis. A P value<0.05 was considered statistically significant. Statistical analyses were performed with SPSS for Wie4ndows, version 12.0 (SPSS, Chicago, IL, USA).
RESULTS
There was no difference in general characteristics between the control group (M:F=7:1, mean age±S.D.; 47.0±10.9 years) and the cirrhosis group (M:F=61:10, mean age±S.D.; 52.0±8.1 years). In patients with liver cirrhosis, no difference in baseline characteristics according to DSE response was observed (Table 1). No complication or adverse event such as arrhythmia or hemodynamic instability related with DSE was observed except a sense of palpitation along with increase of heart rate in eleven patients, however, this symptom was resolved within several minutes after finish of DSE and discontinuation of dobutamine infusion and there was no relationship between the development of palpitation and the results of DSE.
Echocardiographic indices according to DSE outcome
No significant difference in echocardiographic indices at baseline and peak dobutamine infusion between control and cirrhosis patients was observed except baseline and peak E/A ratio. Baseline and peak E/A ratio, common diastolic dysfunction markers, were higher in the cirrhosis group than in controls (Table 2, P<0.001). Blunted DSE response was observed in 18 patients (25.4%) in the cirrhosis group, however, the blunted response to DSE was not observed in the control group (Fig. 3). Therefore, cirrhosis patients were divided into two subgroups according to DSE outcome: a normal DSE response group (n=53, 74.6%) or a blunted DSE response group. Baseline EF was significantly higher in the 18 blunted DSE response patients than those of the normal DSE response patients (P<0.05), however, both were within normal range. According to the definition of blunted DSE response, the change of EF, EDV, and ESV from baseline to peak of dobutamine infusion in the blunted response group showed inverse responses compared with the normal response cirrhotic group, and therefore EDV and ESV increased and EF decreased at peak (Table 2, P<0.001). However, no significant difference was observed in other echocardiographic indices including structural parameters between the normal and the blunted DSE response cirrhosis group (Table 2).
Liver profiles according to DSE outcome
There was no significant difference in Child-Pugh score, MELD score, or etiology of cirrhosis between normal and blunted DSE response groups. Hence, no correlation was documented between the severity of cirrhosis and the development of cardiac dysfunction (Table 1).
Chronotropic incompetence
Including all blunted DSE response patients, 88.6% of the cirrhotic patients showed chronotropic incompetence (HRR less than 80%) for dobutamine infusion. No significant correlation between the severity of chronotropic incompetence and DSE response outcome was observed (P>0.05). However, eight cirrhotic patients who showed paradoxical heart rate responses for dobutamine infusion (HRR less than 0%) also showed blunted DSE response.
DISCUSSION
Cardiac output increases whereas systemic vascular resistance and arterial pressure decrease in cirrhosis. Despite the increased basal cardiac output, the cardiac response to physiologic or pharmacologic stimuli is known to be subnormal, a phenomenon called 'cirrhotic cardiomyopathy (CCM)'.3-7 Without firm diagnostic criteria and a screening test, the exact prevalence of CCM remains unknown. In general, CCM does not show overt heart failure at rest due to an 'auto-treating' effect reducing afterload related to peripheral vasodilatation of cirrhosis and compensatory diminution of inhibitory influences such as the cardiac muscarinic system.5,13 Therefore, in general, CCM is clinically not so problematic in a resting state. However, stresses such as liver transplantation, infection, and procedures like the insertion of TIPS, can convert latent to overt heart failure. Indeed, with increasing liver transplantation, CCM-related heart failure is the third-leading cause of death after rejection and infection.7,14 Therefore, the development of a precise screening test and assessment of CCM severity is necessary in the management of cirrhosis.
Diastolic dysfunction is believed to be present in virtually all CCM patients, and a few reports have suggested that CCM could be diagnosed with simple echocardiographic diastolic dysfunction indices such as the E/A ratio even at rest.15 Additionally, the E/A ratio has been suggested recently as predictive marker for survival after TIPS.16 Our study also showed a significant difference in E/A ratio between controls and the total cirrhotic patients group. In addition, baseline and peak E/A ratio increased significantly according to Child-Pugh class as in previous studies, i.e., cirrhotic patients group showed definitive diastolic dysfunction compared with the normal control group.17 However, in the present study, no significant difference in the severity of diastolic dysfunction was seen between subgroups of cirrhosis according to the DSE response. Many previous studies about diastolic dysfunction of CCM have a limitation because they only compared cirrhosis with normal controls. Diastolic dysfunction could be seen almost old ages. Indeed, some authorities contend that some degree of diastolic dysfunction is present in virtually every patient with cirrhosis.18-21 Therefore, estimation of diastolic dysfunction with simple echocardiography is not enough to differentiate true CCM patients from general cirrhotic patients. The most prominent feature of CCM is systolic dysfunction of LV compared with general cirrhotic patients. Hence, a provocative test for cardiac systolic dysfunction in liver cirrhosis patients based on CCM pathophysiology is needed as a screening test. Until now, many attempts to stress the ventricle by a physiological or pharmacological method have been made. In the case of the exercise test, reduced exercise capacity of cirrhotic patients related with aging process or other causes unrelated with cardiac function made it difficult to use as a provocation test.15,22 While some previous studies used dobutamine on cirrhotic patients, it was just used to assess the risk of ischemic heart disease prior to a major operation; it was not proposed to estimate CCM.23,24
Heart cell contractility is mainly determined by the stimulatory β-adrenergic receptor system. When stimulated by norepinephrine or other catecholamines, the β-adrenergic receptor-ligand complex couples with G-protein to stimulate membrane-bound adenylate cyclase, generating cAMP which phosphorylates several proteins leading to intracellular calcium fluxes. The calcium then causes actin-myosin cross-linking, and thus cellular contraction.8,9 The impairment of such stimulatory β-adrenergic receptor signalling pathways is the main pathogenesis of CCM; hence we assumed that ventricular systolic and diastolic function in a CCM patient would be blunted by norepinephrine or other catecholamines stimulation such as dobutamine. Cardiac responses to environmental changes are probably even more important than how heart function at rest. Dobutamine is the most commonly used sympathomimetic agent for pharmacologic stress testing through having its effect of direct inotropic stimulation. This study is the first to apply DSE for differentiating CCM from general cirrhosis. We analyzed blunted and normal response cirrhosis patients according to left ventricular volume response after dobutamine infusion (Fig. 1, 2, 3). The criteria of abnormal LV volume response used in this study has already been proven as an independent prognostic value for cardiac event during major vascular surgery in the department of cardiology.10 In this study, according to these criteria, definitive blunted or inversed responses of LV during DSE were observed in 18 (25%) among 71 cirrhotic patients. Based on action mechanism of dobutamine, impaired cardiac response to dobutamine stimulation may arise from defects in β-adrenergic receptor signalling pathways, implicated in CCM. It suggests that patients with blunted cardiac response to dobutamine may potentially have higher risk of sudden cardiac events, such as heart failure after major operation than those without impaired response. In fact, no characteristic difference in diastolic indices and liver profiles between normal and blunted DSE response group was observed in either the rest or stressed state. In other words, the latent risk of cardiac event cannot be detected with previously established liver profiles such as Child-Pugh score, MELD, or diastolic cardiac indices. Hence, detecting patients with impaired β-adrenergic receptor signal transduction through DSE prior to a major operation could be helpful to prevent unexpected cardiac events.
In conclusion, blunted cardiac response to dobutamine stimulation were frequently observed in cirrhotic patients, which may be attributed to defects in β-adrenergic receptor signalling pathways, which is one of major pathogenesis of CCM.
Acknowledgements
This research was supported by a grant from The Korean Association for The Study of The Liver 2006, and also by a grant from Ministry of Health and Welfare, Republic of Korea (no. A050021).
Abbreviations
CCM
cirrhotic cardiomyopathy
DSE
dobutamine stress echocardiography
TIPS
transjugular intrahepatic portosystemic stent-shunts
EF
ejection fraction
E/A ratio
early/late diastolic flow ratio
EDV
end-diastolic volume
ESV
end-systolic volume
IVSD
interventricular septal diastolic wall thickness
PWD
Left ventricular posterior wall diastolic thickness
HR
heart rate
HRR
heart rate reserves