Clinical features of acute viral hepatitis B in Korea: a multi-center study
Article information
Abstract
Background/Aims
The incidence of Hepatitis B has significantly declined since the introduction of an HBV vaccination program. The aim of this study was to investigate recent clinical features of acute viral hepatitis B (AVH-B) in Korea.
Methods
A total of 2241 patients with acute viral hepatitis were enrolled and their data were collected from nine medical-centers between January 2006 and December 2009.
Results
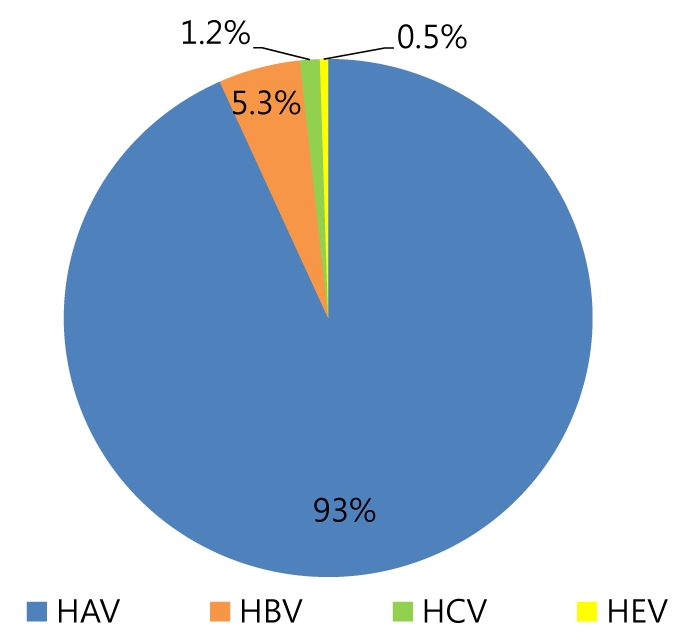
One hundred nineteen (5.3%) of the 2241 were diagnosed as AVH-B. Among 78 patients with AVH-B whose data were analyzed, 50 were male, and the mean age was 38.6 years. In an initial test, mean AST, ALT and total-bilirubin levels were 1296.2 IU/L, 2109.6 IU/L and 9.3 mg/dl, respectively. Positivity frequencies for HBeAg and anti-HBe were 55.1% and 67.9%, respectively, and the mean HBV DNA level was 5.2 log10 copies/ml. The mean length of hospitalization was 11.6 days. During follow-up, AST, ALT and total bilirubin levels were normalized or near-normalized in all patients without serious complications. Sixty-three of 66 (95.4%) patients showed HBsAg loss and 37 (56.1%) patients showed HBsAg seroconversion. Only 3 patients (4.5%) showed persistent hepatitis B viremia. There was no case of death or liver transplantation. Nine patients (11.3%) had received anti-viral agents and their clinical outcomes were not significantly different from those of patients treated without antiviral agents.
Conclusions
The prevalence of AVH-B among acute hepatitis patients is relatively low in Korea. AVH-B infection can be cured without complications in almost all patients, regardless of antiviral treatment.
INTRODUCTION
The incidence of acute viral hepatitis B (AVH-B) in the United States has declined steadily since the late 1980s. The reduction in hepatitis B virus (HBV) incidence in the US may be attributed to effective vaccination programs as well as universal precautions for needle use and in healthcare in general.1 In Korea, an endemic area of HBV, the positivity of hepatitis B surface antigen (HBsAg) also decreased from 8% to 3.7%, since routine HBV vaccination program were introduced.2 Several single-center studies showed that the etiology of acute viral hepatitis in Korea has been changing.3,4 Cases of AVH-B as the etiologic agent in acute viral hepatitis declined in the most recent decade.3 Most primary infections of HBV are self-limited with clearance of virus and development of immunity. However, an estimated 3% to 5% of adults and up to 95% of children develop chronic HBV infection.5 AVH-B can be symptomatic or asymptomatic. In asymptomatic cases, biochemical abnormalities such as elevations of aminotransferase levels are usually undetected unless a routine check-up is performed.6 In symptomatic cases, prodromal symptoms include general malaise, anorexia, nausea, vomiting and fever, which may last for several days to weeks.6 Right upper quadrant discomfort or flu-like symptoms may also present. The patient may then be icteric or unicteric.6 Approximately 1% of persons with AVH-B develop acute liver failure.7 Although the epidemiology has changed, changes in the clinical courses of AVH-B have not been reported in Korea. Therefore, the aim of this study was to analyze data for prevalence and demographic and clinical characteristics of AVH-B in Korea.
PATIENTS AND METHODS
Study population
In this investigator-initiated cohort study, 2241 adult patients were diagnosed with acute viral hepatitis from January 2007 and December 2009 in nine medical centers in Korea. Of the 2241, 119 (5.3%) were diagnosed with AVH-B. Among them, 78 patients whose data could be investigated were included in this study and analyzed retrospectively. The diagnosis of AVH-B was based on HBsAg positivity and/or anti-HBc IgM positivity with acute onset of symptoms including general weakness, abdominal pain, nausea, vomiting and jaundice, etc. No patient had a family history of hepatitis B. Laboratory test results that supported the diagnosis of AVH-B were elevated serum alanine aminotransferase (ALT) and total bilirubin. None of the patients had a previous history of liver disease or reported any other causes of liver injury, such as alcohol or drugs. Patients with diagnoses of chronic hepatitis, HBV carrier, liver cirrhosis, gastric and esophageal varices, or liver cancer were excluded because such cases were considered to be chronic HBV infections. The study protocol was approved by the Institutional Review Board of each hospital and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Laboratory and clinical assessments
Serum ALT levels were measured at baseline, 12 months and final follow-up. HBV serology, including serum hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), hepatitis B e antigen (HBeAg) and antibody to hepatitis B e antigen (anti-HBe), was checked at baseline, 12 months and final follow-up. Serum HBV DNA levels were also assessed using the COBAS Amplicor PCR assay, which has a lower limit of detection of 300 copies/mL (Roche Molecular Systems, Branchburg, NJ, USA). Rates of patients with ALT normalization, HBsAg serologic loss, HBsAg seroconversion and persistent HBV viremia were investigated at final follow-up. Incidence rates of complications, in-hospital mortality and length of stay were also investigated. Accepted criteria for defining clinical and serologic recovery from AVH-B are clearance of circulating HBsAg and appearance of anti-HBs, with normalization of serum aminotransferase levels. We analyzed clinical profiles of groups treated with antiviral agents compared with those of groups treated without antiviral agents.
Statistical analysis
HBV DNA levels were logarithmically transformed for analysis. Continuous variables were expressed as the mean±standard deviation (SD) and categorical variables as absolute and relative frequencies. The χ2 test was used to analyze categorical variables such as gender and hepatitis serology including HBsAg, anti-HBs, HBeAg, and anti-HBe. Comparisons of continuous variables, such as age, serum HBV DNA level, and serum ALT level, were analyzed using a t-test. P-values less than 0.05 were considered statistically significant. SPSS version 17.0 was used for all statistical analysis (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics of the study population
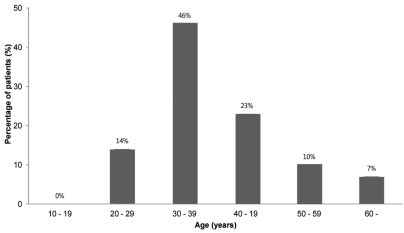
A total of 2241 patients were diagnosed with acute viral hepatitis, and 119 (5.3%) of them were diagnosed with AVH-B (Fig. 1). Among the 119 AVH-B patients, 78 whose data could be investigated were enrolled. The baseline characteristics of the patients are shown in Table 1. The mean age of patients was 38.6 years and the highest rate corresponds to the group aged 30-39 years (Fig. 2). Thirty-one (40%) of 78 patients were more than 40 years old.
Clinical, biochemical and virologic profiles at the onset of AVH-B
All patients were HBsAg positive and IgM anti-HBc positive at presentation. Ten patients were anti-HBs positive. Forty three (55.1%) patients were HBeAg positive and 53 (67.9%) patients were anti-HBe positive. The peak ALT levels ranged from 52 to 5477 IU/L, and the peak total bilirubin levels ranged from 0.8 to 33.8 mg/dL. The mean peak HBV DNA level were 5.2 log10 copies/mL and peak HBV DNA levels ranged from undetectable to 8.2 log10 copies/mL (Table 1). Mean duration of hospitalization was 11.6 days.
Clinical, biochemical, and serologic profiles at follow-up
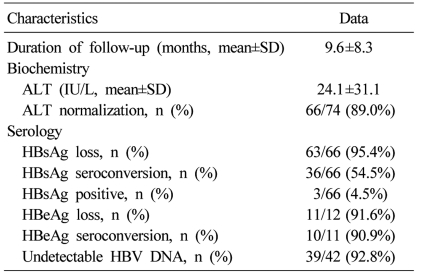
Seventy four patients were examined for clinical and serologic recovery (Table 2). Mean duration of follow-up was 9.6 months. At the final follow-up, serum ALT levels were within normal limits in 66 (89%) of the 74 patients. Sixty six of the 74 patients were examined for serologic markers for HBV. Sixty three (95.4%) of the 66 patients showed serologic loss of HBsAg, and 36 (54.5%) of the 66 patients showed seroconversion of HBsAg. After final follow-up, 3 (4.5%) out of the 66 patients showed persistent hepatitis B viremia (Table 3). Three patients had follow-up for 9 to 18 months. They all showed normalization of serum ALT levels but persistent viremia. Symptoms related to AVH-B improved in all patients. There was no case of progression to fulminant hepatitis, death or liver transplantation.
Antiviral treatments and clinical courses
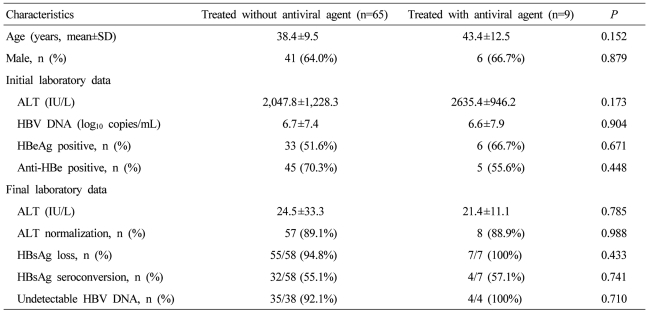
Nine (11.5%) patients among the 74 patients tested for serologic markers during the follow-ups had received antiviral agents (lamivudine for 3, entecavir for 4, and clevudine for 2). The mean duration of treatment was 10.3 months. At final follow-up, all patients showed normalization of ALT levels. Only 7 were examined for HBV serologic marker at final follow-up and all showed HBsAg serologic loss. Four (57.1%) showed seroconversion of HBsAg. Sixty five patients were not treated with antiviral agents. Fifty five (94.8%) of 58 were examined for HBV serologic markers at final follow-up and showed HBsAg serologic loss. Thirty two (55.1%) of the 58 patients showed HBsAg seroconversion (Table 4). HBV clearance rates were not significantly different between the groups with and without antiviral treatment.
DISCUSSION
This study showed that the prevalence of AVH-B among acute viral hepatitis patients is relatively low, and that after the introduction of the HBV vaccination program in Korea the age group most susceptible to AVH-B had changed from people aged 10-29 years to people aged 30-39 years.
Worldwide, two billion people have been infected with hepatitis B virus infection; 360 million have a chronic infection; 600,000 die each year from HBV-related liver disease or hepatocellular carcinoma.8 Universal hepatitis B vaccination for all infants, which was introduced in the early 1980s, is now widely accepted as a fundamental strategy to protect the general public from this potentially severe disease.8 Systemic immunization is the most cost-effective tool in preventing the spread of HBV infection.9 A study conducted in Taiwan showed that since the introduction of the infant HBV immunization program in 1984, the prevalence rates of HBV infection in children under age 15 were reduced from 9.8% in 1984 to 1.3% in 1991.10 In Korea, vaccination against HBV has been provided for infants born to mothers with HBV since 1983. Before the introduction of the HBV vaccination program, approximately 8% of the general Korean population tested positive for HBsAg.11 The rate of HBsAg carriage in the general population had decreased to 3.7% by 2007.
The majority of acute hepatitis B cases in the early 1980s occurred in people aged 10-29 years.12 The decline in the incidence of acute hepatitis B after the introduction of the HBV vaccination program was more striking in adults aged 10-29 years; these are individuals who were born after the introduction of HBV vaccination.12 The incidence of acute hepatitis B has been changing in the early 2000s: it was highest among adults aged 30-39 years, who were born before the introduction of the HBV vaccination program.4,12 In this multicenter study, the mean age of patients was 38.6 years and the highest rate corresponds to the group aged 30-39 years. The age distribution of acute hepatitis B in our study was similar to that of a recent report by Yim et al.4 Therefore, unvaccinated and uninfected adults may be vulnerable to AVH-B and require catch-up vaccination to protect against HBV infection.
Approximately 5% of all acute HBV infections progress to chronic infection, and the risk of progression from an acute to chronic phase is inversely proportional to age.13 In the acute phase of HBV infection, the manifestations range from subclinical hepatitis to symptomatic hepatitis requiring hospitalization. The most serious form of presentation of acute hepatitis is fulminant hepatic failure with a fatality rate of 70%.14 One study on the incidence of fulminant hepatitis in acute viral hepatitis B patients showed the incidence of fulminant hepatitis was 6.0%.15 In this study, the symptoms of AVH-B improved in all patients, and there was no case of progression to fulminant hepatitis, death, and liver transplantation. Patients of tertiary hospitals were enrolled in this study and there was the possibility of including severe AVH-B cases, but the prognosis of enrolled patients was good.
In primary infection, HBsAg becomes detectable after 4 to 10 weeks of incubation, followed by the presence of antibodies against the core antigen (anti-HBc) in IgM during the early period.16 Viremia is well established by the time HBsAg is detected. Once this response has commenced, viral titers in blood and liver begin to drop. HBsAg is the first marker to appear in the blood. HBeAg may appear early but is cleared rapidly. HBsAg is cleared and its antibody, anti-HBs, appears within 6 months of disease onset in most cases. The outcome of acute hepatitis B is usually good with complete recovery from the liver damage and seroconversion to anti-HBs, which represents a long-term protection for HBV infection.6 However, when persistent infection becomes established, serology markers like HBeAg, anti-HBe and anti-HBs can be positive or negative except HBsAg and anti-HBc IgG remain positive.4 Yuki et al17 showed that occult HBV infection persists in the liver and is accompanied by abnormal liver histology for a decade after complete clinical recovery from acute self-limited hepatitis B. In the present study, 63 (95.4%) of 66 patients showed serologic loss of HBsAg and 36 (54.5%) of 66 patients showed seroconversion of HBsAg. After final follow-up, 3 (4.5%) of 66 patients showed persistent hepatitis B viremia. The rate of chronicity of AVH-B in the present study was similar to that in a previous report.17
Uncomplicated, self-limited acute HBV requires no specific treatment. Some data have indicated a possible role for lamivudine treatment of selected patients with severe or fulminant acute HBV infection. Kumar, et al showed that though lamivudine causes a greater decrease in levels of HBV DNA, it does not cause significantly greater biochemical and clinical improvement as compared to placebo in patients with acute hepatitis B.18 In another study, early treatment with lamivudine led to a greater decrease in HBV DNA levels, better clinical improvement and lower mortality in patients with severe acute hepatitis B.19 In our study, we compared HBsAg seroconversion rates, serum HBV DNAnegative rate, biochemical indicators, the incidence of liver failure and mortality between one group treated with antiviral agents and the other group without antiviral agents. The clinical outcomes between the two groups were not significantly different. One reason for the lack of significant difference could be that no patient suffered from persistent hepatitis B viremia in the group treated with antiviral agent. Limitations of our study include a small number of subjects and the retrospective design. Therefore, further evidence for antiviral agents in acute hepatitis B and fulminant hepatitis is required.
In conclusion, in this multi-center study, the prevalence of AVH-B among acute viral hepatitis patients is relatively low in Korea. The prognosis of patients with AVH-B is good in the majority of enrolled patients. However, some patients progress to a chronic state regardless of antiviral treatment. Catch-up HBV vaccinations in adult population may be required to protect against HBV infection in Korea.
Acknowledgements
This research was supported by the GlaxoSmithKline Research Fund of the Korean Association for the study of the Liver.
Abbreviations
ALT
alanine aminotransferase
anti-HBc
antibody to hepatitis B core antigen
anti-HBe
antibody to hepatitis B e antigen
anti-HBs
antibody to hepatitis B surface antigen
AST
aspartae transaminase
AVH-B
acute viral hepatitis B
DNA
deoxyribonucleic acid
HBeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
PCR
polymerase chain reaction