Favorable effect of corticosteroids in treating acute-on-chronic liver failure underlying chronic hepatitis B
Article information
Abstract
Acute-on-chronic liver failure (ACLF) occurs in the presence of a chronic liver disease or cirrhosis, and often results from exacerbation of chronic hepatitis B (CHB). The efficacy of corticosteroid treatment in ACLF patients with underlying CHB remains unclear. We report the case of a 50-year-old woman who experienced ACLF due to CHB exacerbation and was treated with a combination of corticosteroids and nucleot(s)ide analogue (NUC). The patient showed rapid decompensation due to CHB exacerbation. Three months of antiviral therapy produced no improvement in liver function. Combination therapy with corticosteroids and NUC was started, which did result in improvement of liver function. This case shows that the combined therapy of corticosteroids and NUC can be effective in treating ACLF due to CHB exacerbation.
INTRODUCTION
Acute-on-chronic liver failure (ACLF) has emerged as a distinct clinical entity and manifests as liver failure in the presence of chronic liver disease or cirrhosis. An acute insult such as exacerbation of chronic hepatitis B (CHB), alcohol consumption, drug-induced liver injury and other viral hepatitis can lead to ACLF.1 The pathophysiology of ACLF relates to persistent inflammation, immune dysregulation with a systemic inflammatory response syndrome and subsequent sepsis due to immune paresis [1].
Acute exacerbation of CHB can lead to fatal liver failure characterized by a high alanine aminotransferase level, jaundice, and coagulopathy [2]. The use of nucleot(s)ide analogues (NUCs) can be effective in managing CHB exacerbation, but their efficacy in preventing rapid progression to ACLF is limited [3]. The main pathogenesis of CHB exacerbation is considered to be liver injury through the induction of cytotoxic T-lymphocyte-mediated cytolytic pathways in hepatitis B virus (HBV)-infected hepatocytes [4]. Combined administration of corticosteroids with NUCs may counter liver function deterioration in patients with ACLF and can inhibit the immune-mediated reaction of T-lymphocyte, thus preventing cytolysis of infected hepatocytes [5]. However, in some cases, the corticosteroid treatment may enhance replication of HBV DNA, aggravating the condition, and lead to fulminant hepatic failure. Therefore, the efficacy of corticosteroid in managing acute exacerbation of CHB is unclear.
Here, we report a case of successful treatment of ACLF with underlying CHB with combined with corticosteroids and NUC therapy.
CASE REPORT
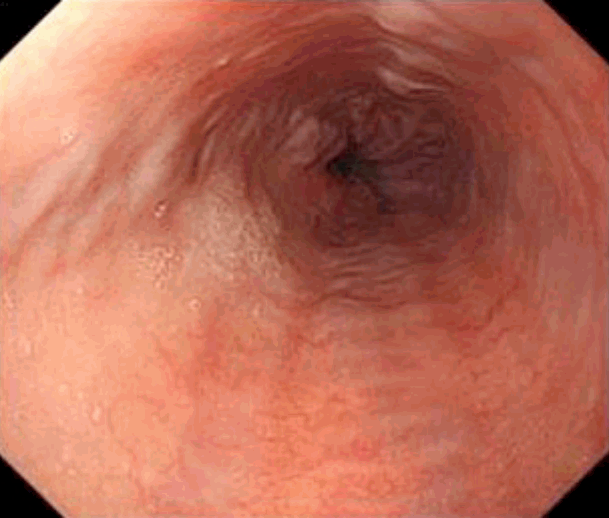
A 50-year-old woman was referred to our hospital for liver transplantation. She was diagnosed with CHB approximately 20 years ago, but was not receiving any antiviral treatment. Before being referred to our hospital, she was admitted to another hospital due to CHB flare up (Fig. 1A). She denied alcohol consumption and taking no other medications or herbal supplements. She was treated with tenofovir 300 mg daily. However, despite one-month treatment with tenofovir, newly developed ascites were seen and jaundice progressed; the total bilirubin level elevated 4 times higher than initial value. After that, she kept treatment with medication and underwent intermittent abdominal paracentesis for 2 months. Because there was no improvement of liver function, she referred to our hospital. At the time of admission to our hospital, she had a blood pressure of 100/70 mmHg; pulse rate, 103 beats/min; respiration rate, 20 breaths/min; and temperature, 36.6°C, with a clear consciousness. Physical examination showed icteric sclerae, a distended abdomen without tenderness, and pitting edema. Results of laboratory tests on admission were as follows: albumin level, 2.4g/dL (3.8-5.1 g/dL); total bilirubin level, 15.4 mg/dL (0.2-1.4 mg/dL); direct bilirubin level, 7.9 mg/dL (0-0.3 mg/dL); alkaline phosphatase level, 196 IU/L (38-110 IU/L); gamma- glutamyl transpeptidase level, 36 IU/L (0-38 IU/L); aspartate aminotransferase level, 113 IU/L (9-40 IU/L); alanine aminotransferase level, 61 IU/L (9-40 IU/L); hemoglobin level, 12.1 g/dL (12- 15 g/dL); white blood cell count, 8.94×103/μL (4-9.9×103/μL); platelet count, 96×103/μL (140-400×103/μL); creatinine level, 0.9 mg/dL (0.5-1.2 mg/dL); and prothrombin time (PT), 17.7 seconds (10.4-12.9 seconds), with a calculated Child-Pugh score of 11 (C) and a model for end-stage liver disease (MELD) score of 20. HBeAg test result was positive, while anti-HBe test result was negative. HBV DNA level was already less than 20 IU/mL (Table 1). Serologic tests for immunoglobulin M (IgM) anti-hepatitis A virus, anti-hepatitis C virus (HCV) antibody, HCV real-time polymerase chain reaction, human immunodeficiency virus (HIV) antigen, anti-HIV antibody, IgM anti-cytomegalovirus, IgM anti-Epstein-Barr virus viral-capsid antigen were negative. C-reactive protein level was 5.55 mg/L (0-5 mg/L). Ascites study revealed that serum-ascites albumin gradient was 2.2 g/dL and absolute neutrophil count was 10 cells/μL. Dynamic contrast-enhanced computed tomography (CT) showed a cirrhotic configuration of the liver with mild splenomegaly and moderate amount of ascites (Fig. 1B). Esophagogastroduodenoscopy revealed grade 1 esophageal varix (Fig. 2). While waiting for a cadaveric liver donor, she underwent abdominal paracentesis once or twice a week. The total bilirubin level and PT did not improve but remained unchanged. Her performance status remained at grade 1.
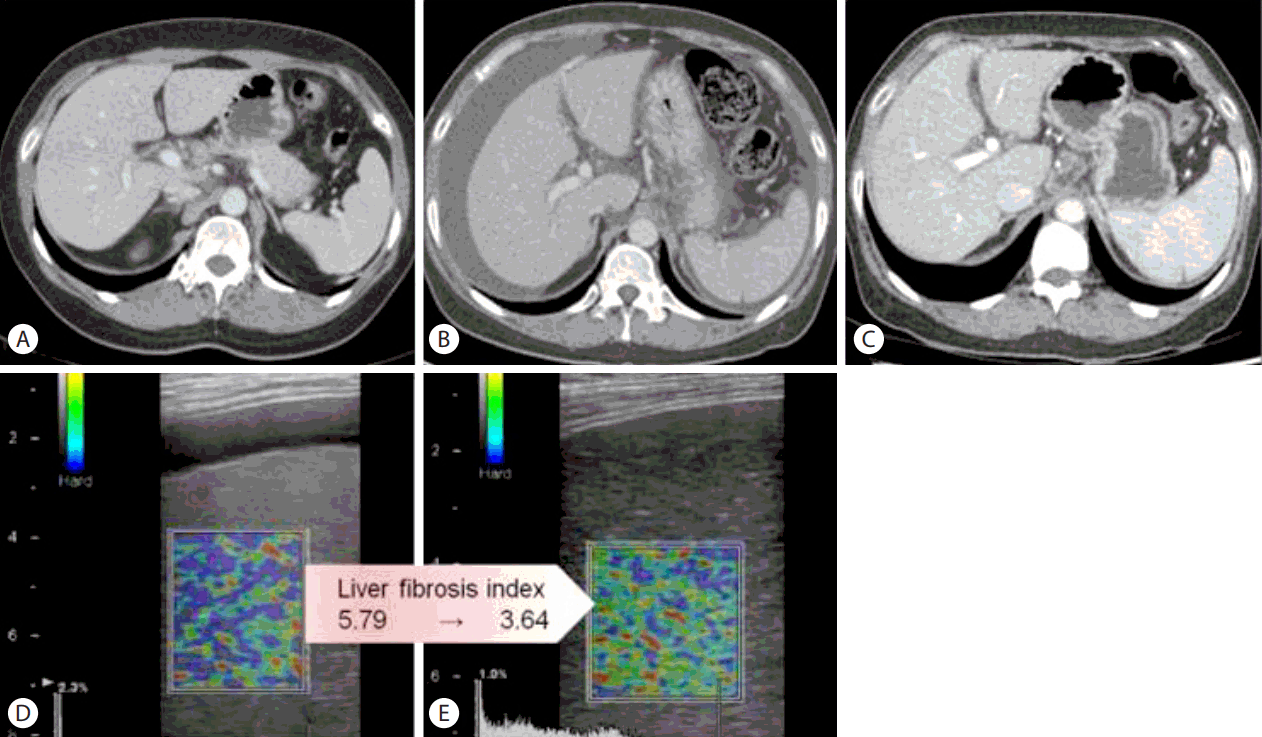
Dynamic contrast-enhanced computed tomography (CT) images in the portal venous phase (A–C) and real-time elastography (RTE) images (D, E). (A) CT scan obtained 3 months before admission to our hospital showing a cirrhotic liver. (B) Upon admission to our hospital, a moderate amount of ascites was observed due to acute-on-chronic liver failure (ACLF) with chronic hepatitis B (CHB). (C) CT scan showing that combined treatment with a corticosteroid and a nucleot(s)ide analogue (NUC) decreased the amount of ascites. (D, E) RTE images obtained after combined treatment show an improved liver fibrosis index.
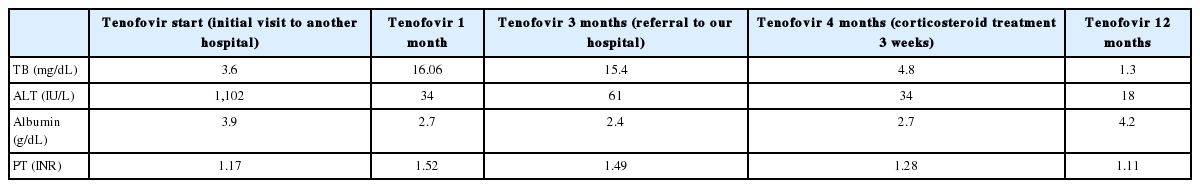
Serial levels of laboratory parameters before and after combined treatment with a nucleot(s)ide analogue and a corticosteroid
To rule out other concomitant causes of liver function deterioration, further evaluation for autoimmunity was performed. The anti- nuclear antibody (ANA) test result was weakly positive, at a titer of 1:80. The anti-mitochondrial antibody, anti-smooth muscle antibody and anti-liver kidney microsome antibody-1 test results were negative, and immunoglobulin G level was 1,338.1 mg/dL (800-1,800 mg/dL). Although we considered percutaneous liver biopsy, we couldn’t do it because bleeding risk was higher due to PT prolongation and moderate amount of ascites.
Because there was no improvement in liver function despite treatment with NUC for 3 months and liver donor was unavailable, prednisolone was started after careful consideration. Although the patient did not show a definite evidence of autoimmunity, we speculated that she had excessive immune activity due to ACLF with CHB. After confirming that HBV DNA was undetectable and chest radiography and urinalysis showed no evidence of infection, prednisolone therapy was started at 30 mg/day.
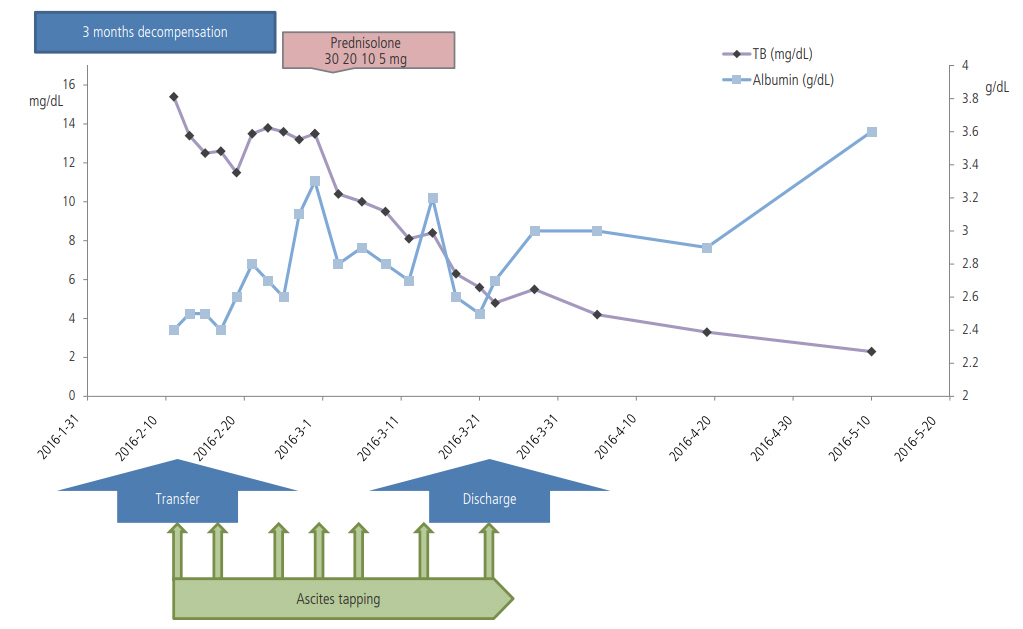
Shortly after combination therapy with corticosteroid and NUC, resolution of ACLF was seen. Total bilirubin level decreased by 2 to 5 mg/dL every 7 days, decreasing from 15.4 mg/dL to 5.6 mg/dL in only 3 weeks (Fig. 3). Prednisolone dose was tapered by 5 or 10mg at least every 4 days, to 5 mg, depending on the decreasing trend of the total bilirubin level. Prednisolone therapy was subsequently stopped. The patient was discharged on tenofovir and oral diuretics 1 week after the cessation of combination therapy. Abdominal paracentesis was not needed after discharge. On one-year follow-up, her Child-Pugh score and MELD score improved to 5(A) and 4, respectively. The amount of ascites decreased on follow-up CT (Fig. 1C). Her elastography scores for liver fibrosis also improved from 5.79 to 3.64 (Fig. 1D, E). During the follow-up period, the ANA converted negative on 6 months after admission.
DISCUSSION
Up to 30% of patients with CHB experience spontaneous reactivation of hepatitis every year, and up to 8% of patients develop decompensation, that is, severe acute exacerbation, and may progress to ACLF [1,6]. Reactivation of HBV could be due to changes in the immunological control of viral replication and reconstitution of host defenses [1]. The prognosis of patients with severe acute exacerbation of CHB leading to ACLF is extremely poor [2].
In acute exacerbation of CHB, administration of NUCs results in rapid cessation of HBV DNA replication and thus reduced HBV DNA level. We used tenofovir as the NUC in this case. A randomized controlled trial showed that reducing the HBV DNA level by >2logs from baseline within 2 weeks using tenofovir improved the transplant-free survival rate [7]. However, the improvement in liver function with administration of NUCs may take a few weeks to a few months, during which period excessive immune activity may continue and liver cell injury may progress [8]. The corticosteroid can modulate the activity of CHB during this period by suppressing the host immune response to HBV antigens [5]. Therefore, administering corticosteroids to patients with ACLF with underlying CHB is a reasonable treatment decision [9]. A recent meta-analysis showed that treatment with corticosteroids can significantly increase the survival rate of patients with severe hepatitis B infection. 10 This study showed that the corticosteroid treatment is more effective in patients at the pre-liver failure stage and is not associated with the development of secondary infection and bleeding.
However, reactivation of the HBV in patients with malignant, inflammatory and autoimmune conditions who undergo immunosuppression has been widely reported. Higher HBV reactivation rates have been observed in patients with hematological malignancies receiving treatment regimens that utilize high-dose corticosteroids and/or rituximab [11,12]. Patients who are HBsAg negative/hepatitis B core antibody positive also remain at risk of HBV reactivation in the setting of immunosuppression owing to the persistence of the HBV in the form of cccDNA in hepatocytes and other tissues [13], and long-term use of corticosteroids particularly increases the risk of HBV reactivation [10]. Therefore, administration of NUCs for HBV replication inhibition combined with a short-term corticosteroid therapy should be considered in such risky patients [3,8,14].
Several studies have reported on the importance of early introduction of corticosteroid treatment in ACLF with CHB [3,8,9], as delayed introduction, for example, after 10 days, may not be effective because a large number of hepatocytes might have already been destroyed and inhibition of the inflammatory reaction might not be effective [3,8]. However, “early stage” or “pre-liver failure” in CHB exacerbation has not yet been clearly defined. In this case, corticosteroid therapy was administered after 3 months of NUC treatment and maintained for 3 weeks.
Our patient maintained a good performance status and liver function without further deterioration while waiting for the liver donor. This indicated that not all hepatocytes of the patient were destroyed. Furthermore, the ANA test showed weak positivity; false positivity in sometimes seen in cases of CHB exacerbation. We suspected combined autoimmunity in our patient. Therefore, a moderate corticosteroid dose was used, unlike in reported studies, whereby an initial dose of 40 mg or more daily prednisolone was administered without evidence of autoimmune hepatitis (AIH) [8-10,15,16]. There have been several case reports of AIH developed as part of immune reconstitution inflammatory syndrome after highly active antiretroviral therapy (HAART) in HIV infected patients [17]. The mechanism that results in AIH after initiation of HAART in HIV patient is probably involves changes in CD4+ T cells [18]. The rapid increase in CD4+ T cells may produce a larger proportion of Th17 cells that release IL-17 without appropriate Treg cells numerical and functional reconstitution, which are similar to AIH. Infiltration of CD 4+ T cells in the liver are increased in patients with AIH and IL-17 signaling pathway play a critical role in the pathogenesis of AIH. Likewise, it is possible to hypothesize that immune dysregulation caused by ACLF was modulated after NUC treatment and subsequent autoimmunity was induced by restored immunity. It suggests that immune reconstitution after NUC treatment had a role for good response to corticosteroid treatment although the patient was treated with delayed introduction after the diagnosis of CHB exacerbation.
In our patient, no newly developed infection, bleeding, or HBV reactivation was seen during corticosteroid treatment. HBV DNA suppression was enough adequate and the possibility of infection was ruled out before initiating the corticosteroid treatment. Adverse effects of corticosteroid combination therapy such as pneumonia or enteritis have been reported [2], but there are no reports on HBV reactivation.
In conclusion, this case suggests that combined corticosteroid and NUC therapy may be effective in patients with ACLF with CHB, particularly in those with suspected autoimmunity. However, a careful consideration of patient condition prior to initiation and monitoring for infection are necessary.
Notes
Authors’ contribution
Jung Hyun Kwon planed the study and reviewed the references. Hyeji Kim and Jung Hyun Kwon wrote the paper. Yong Hee Kim, Soon Woo Nam and Jong Yul Lee treated and reviewed the patient. Jeong Won Jang gave a comment for consultation.
Conflict of Interest
The authors have no conflicts to disclose.
Abbreviations
ACLF
acute-on-chronic liver failure
ANA
anti-nuclear antibody
AIH
autoimmune hepatitis
CHB
chronic hepatitis B
CT
computed tomography
HAART
highly active antiretroviral therapy
HBV
hepatitis B virus
HCV
hepatitis C virus
HIV
human immunodeficiency virus
IgM
immunoglobulin M
MELD
model for end stage liver disease
NUC
nucleot(s)ide analogue
RTE
real-time elastography
PT
prothrombin time