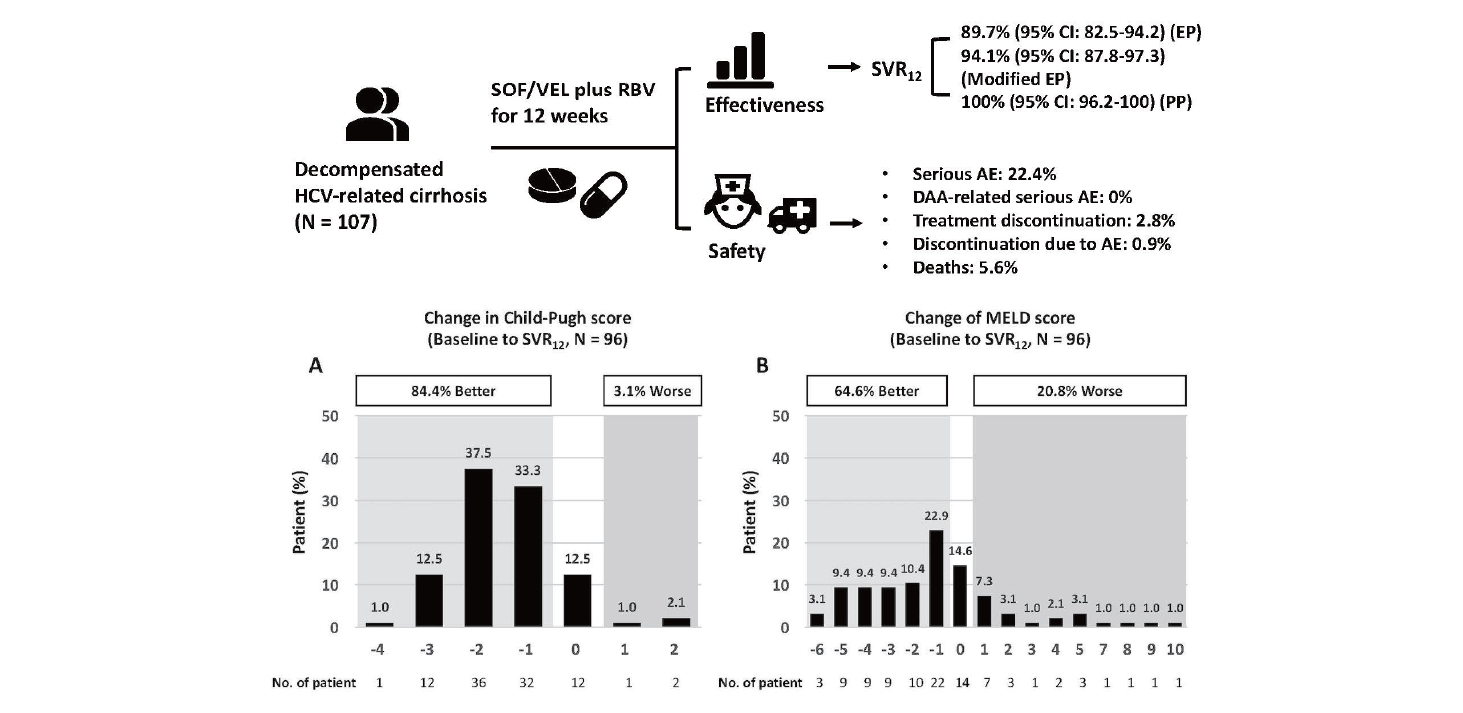
Sofosbuvir/velpatasvir plus ribavirin for Child-Pugh B and Child-Pugh C hepatitis C virus-related cirrhosis
Article information
Abstract
Background/Aims
Real-world studies assessing the effectiveness and safety of sofosbuvir/velpatasvir (SOF/VEL) plus ribavirin (RBV) for Child-Pugh B/C hepatitis C virus (HCV)-related cirrhosis are limited.
Methods
We included 107 patients with Child-Pugh B/C HCV-related cirrhosis receiving SOF/VEL plus RBV for 12 weeks in Taiwan. The sustained virologic response rates at off-treatment week 12 (SVR12) for the evaluable population (EP), modified EP, and per-protocol population (PP) were assessed. Thesafety profiles were reported.
Results
The SVR12 rates in the EP, modified EP and PP were 89.7% (95% confidence interval [CI], 82.5–94.2%), 94.1% (95% CI, 87.8–97.3%), and 100% (95% CI, 96.2–100%). Number of patients who failed to achieve SVR12 were attributed to virologic failures. The SVR12 rates were comparable regardless of patient characteristics. One patient discontinued treatment because of adverse events (AEs). Twenty-four patients had serious AEs and six died, but none were related to SOF/VEL or RBV. Among the 96 patients achieving SVR12, 84.4% and 64.6% had improved Child-Pugh and model for end-stage liver disease (MELD) scores. Multivariate analysis revealed that a baseline MELD score ≥15 was associated with an improved MELD score of ≥3 (odds ratio, 4.13; 95% CI, 1.16–14.71; P=0.02). Patients with chronic kidney disease (CKD) stage 1 had more significant estimated glomerular filtration rate declines than patients with CKD stage 2 (-0.42 mL/min/1.73 m2/month; P=0.01) or stage 3 (-0.56 mL/min/1.73 m2/month; P<0.001).
Conclusions
SOF/VEL plus RBV for 12 weeks is efficacious and well-tolerated for Child-Pugh B/C HCV-related cirrhosis.
Graphical Abstract
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is the leading cause of cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation. The annual incidence of hepatic decompensation ranges from 3% to 6% in HCV-infected patients with established cirrhosis [1]. If HCV is left untreated, the probability of survival after the diagnosis of decompensated cirrhosis is 81.8% and 50.8% at 1 and 5 years, respectively. Compared to patients with Child-Pugh B cirrhosis, the health-related outcome is further compromised in patients with Child-Pugh C cirrhosis [2]. Although liver transplantation provides a survival benefit for decompensated HCV-related cirrhosis, donor organ shortages may prevent timely intervention [3]. In addition to universal HCV recurrence following liver transplantation, the post-transplantation course in recipients is accelerated, with 30% of them developing cirrhosis and graft loss within 5 and 1 years, respectively [4,5]. In contrast, HCV cure by effective antiviral therapies is associated with improved liver reserve, increased survival, and decreased serious liver-related events [6-10].
The advent of interferon (IFN)-free direct-acting antivirals (DAAs) has led to a paradigm shift in the care of HCV. This is particularly relevant for patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis because IFN-based therapies are contraindicated in these patients. Although various DAA regimens have been approved for treating HCV, treatment regimens comprising a protease inhibitor are contraindicated for patients with Child-Pugh B and Child-Pugh C cirrhosis because of the potential risks of life-threatening liver injuries [11-13].
The phase 3 ASTRAL-4 trial compared the performance of sofosbuvir/velpatasvir (SOF/VEL) alone for 12 or 24 weeks, and SOF/ VEL plus weight-based ribavirin (RBV) for 12 weeks in HCV genotype (GT) 1–6 patients with Child-Pugh B HCV-related cirrhosis. The sustained virologic response rate at off-treatment week 12 (SVR12) in participants receiving SOF/VEL plus weight-based RBV for 12 weeks was 94%, which was superior to the SVR12 rates of 83% and 86% in participants receiving SOF/VEL alone for 12 and 24 weeks, respectively [14]. Furthermore, most patients tolerated treatment well. An extended study to assess the efficacy of SOF/VEL plus weight-based RBV for 12 weeks in 23 patients with Child-Pugh C HCV-related cirrhosis revealed that the SVR12 rate was 70%. Among the 16 patients who completed off-therapy follow-up, all patients achieved SVR12 [15]. Based on the excellent clinical performance and pan-genotypic potency, the guidelines have recommended the use of SOF/VEL plus RBV for 12 weeks to manage Child-Pugh B and Child-Pugh C HCV-related cirrhosis [11-13].
To date, real-world studies assessing the effectiveness and safety of SOF/VEL plus RBV in Child-Pugh B and Child-Pugh C HCV-related cirrhosis are limited, with only one report from China showing the SVR12 rates of 100% in 12 patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis receiving SOF/VEL plus RBV for 12 weeks [16]. To this end, we aimed to assess the effectiveness and safety of SOF/VEL plus RBV for 12 weeks in a real-world multicenter practice for Child-Pugh B and Child-Pugh C HCV-related cirrhosis.
MATERIALS AND METHODS
Patients
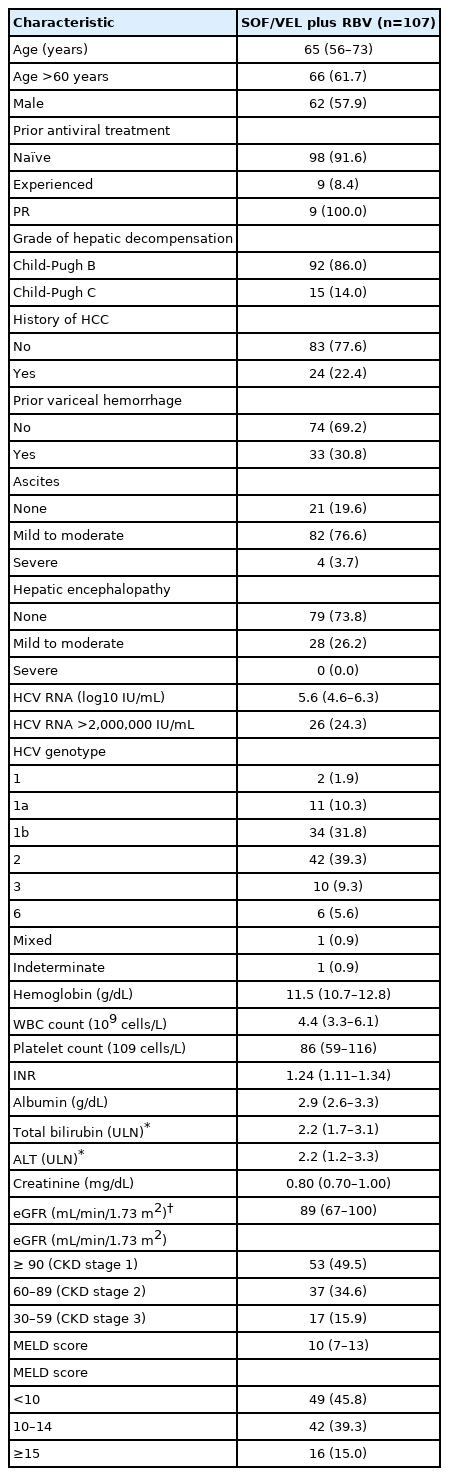
Between July 2019 and June 2020, patients aged ≥20 years who had chronic Child-Pugh B or Child-Pugh C HCV-related cirrhosis and who were scheduled to receive SOF/VEL plus RBV for 12 weeks were retrospectively included at 15 academic centers in Taiwan. Chronic HCV infection was defined as the presence of serum HCV antibody (anti-HCV, Abbott HCV EIA 2.0; Abbott Laboratories, Abbott Park, IL, USA) and quantifiable serum HCV ribonucleic acid (RNA) (Cobas TaqMan HCV Test v2.0; Roche Diagnostics GmbH, Mannheim, Germany; lower limit of quantification [LLOQ], 15 IU/mL) for ≥6 months. Child-Pugh B cirrhosis was defined as patients with a Child-Pugh score of 7 to 9, and Child-Pugh C cirrhosis was defined as patients with a Child-Pugh score of 10 to 15 [17]. Patients who had active HCC, had confirmed virologic failures to prior NS5A-containing DAA regimen, had undergone liver transplantation, were co-infected with hepatitis B virus (HBV) defined as the presence of serum HBV surface antigen (HBsAg) (Abbott Architect HBsAg qualitative assay; Abbott Laboratories) or human immunodeficiency virus (HIV) defined as the presence of serum HIV antibody (anti-HIV, Abbott Architect HIV Ag/Ab Combo; Abbott Laboratories), had other liver diseases such as autoimmune hepatitis or primary biliary cholangitis, or had chronic kidney disease (CKD) stage 4 or 5 defined as an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, were excluded from the study [18]. The study was approved by the Research Ethics Committee of each participating center and was conducted in accordance with the principles of Declaration of Helsinki in 1975.
Study design
Baseline demographics, hemogram, international normalized ratio (INR), serum albumin, total bilirubin (upper limit of normal [ULN], 1.0 mg/dL), direct bilirubin, aspartate transaminase (AST), alanine transaminase (ALT) (ULN, 30 U/L for males and 19 U/L for females), creatinine, eGFR as assessed by the CKD-EPI equation, anti-HCV, HBsAg, anti-HIV, HCV RNA, HCV GT (Roche Cobas HCV GT; Roche Diagnostics GmbH or Abbott RealTime HCV Genotype II; Abbott Laboratories) were collected for all patients [19,20]. Hepatic imaging studies, including ultrasonography, computed tomography and magnetic resonance imaging, were also performed to confirm the presence of HCC or ascites. The Child-Pugh and model for end-stage liver disease (MELD) scores were calculated for all patients [17,21].
All patients received SOF/VEL (Epclusa®, fixed-dose combination 400/100 mg per tablet; Gilead Sciences, Carrigtohill, Ireland) 1 tablet once daily plus RBV (Robatrol®, 200 mg per capsule; Genovate Biotechnology Co. Ltd., Hsinchu, Taiwan) for 12 weeks. The dose of RBV was prescribed according to the label recommendation (1,200 mg daily for body weight ≥75 kg, and 1,000 mg daily for body weight <75 kg in patients with an eGFR ≥50 mL/min/1.73 m2; 200 mg and 400 mg every other day in patients with an eGFR between 30–49 mL/min/1.73 m2 regardless of body weight) [22]. The dose modification of RBV following treatment was based on physicians’ discretion. All patients underwent monitoring at on-treatment weeks 4, 8, and 12 and at off-treatment week 12 according to the regulations of the National Health Insurance Administration of Taiwan. Hemogram, total bilirubin, direct bilirubin, AST, ALT, creatinine and eGFR were assessed at on-treatment weeks 4, 8, and 12. Hemogram, INR, albumin, total bilirubin, direct bilirubin, AST, ALT, creatinine, and eGFR were assessed at off-treatment week 12. Serum HCV RNA levels were assessed at on-treatment week 12 and off-treatment week 12. Hepatic imaging studies were assessed at off-treatment week 12 and were performed at other time points if necessary.
Drug adherence
Drug adherence was presented as the percentage of the total pills consumed during treatment divided by the dispended pills for SOF/VEL and RBV, respectively, in patients who completed 12 weeks of treatment.
Effectiveness
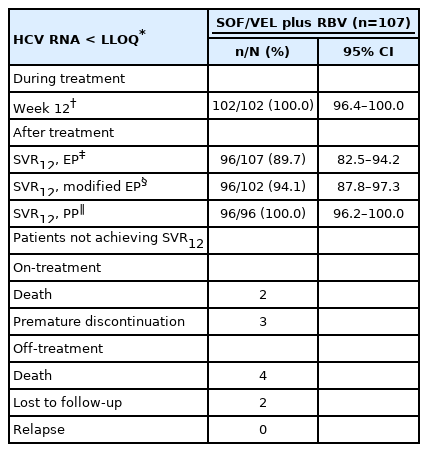
The end-of-treatment virologic response at on-treatment week 12 and sustained virologic response and off-treatment week 12 were assessed. Patients were judged not to achieve SVR12 if the HCV RNA level was > LLOQ at off-treatment week 12 (virologic failure) or if patients had missing SVR12 data (non-virologic failure). The SVR12 endpoints included the evaluable population (EP) for patients receiving at least one dose of SOF/VEL or RBV, the modified evaluable population (modified EP) for patients completing a 12-week course of SOF/VEL plus RBV treatment, and the per-protocol population (PP) for patients with available SVR12 data.
Safety
In patients who prematurely discontinued treatment or who had serious adverse events (AEs) including death, life-threatening condition, inpatient hospitalization, persistent disability, or important medical events requiring emergent intervention, we reviewed the medical records and assessed the causal relationship between HCV medications and these events. We also reported common AEs with rates of ≥10%, and the proportions of patients with ≥ grade 2 anemia and ≥ grade 3 leukopenia, thrombocytopenia, total bilirubin and ALT elevations according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [23]. The doses of RBV following antiviral treatment were shown according to on-treatment hemoglobin levels.
Statistical analysis
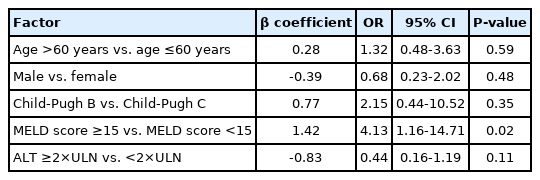
We used the Statistical Program for Social Sciences (SPSS Statistics version 23.0; IBM Corp., Armonk, NY, USA) for all statistical analyses. Baseline characteristics were presented as median (interquartile range, IQR) and numbers (percentages). The virologic response rates at on-treatment and off-treatment weeks 12 were presented as numbers (percentages) with 95% confidence intervals (CIs). The subgroup analysis of SVR12 in EP was presented as number (percentage) with 95% CI. Common AEs and laboratory abnormalities were presented as numbers (percentages). We analyzed the changes in Child-Pugh and MELD scores from baseline to off-treatment week 12 in patients who achieved SVR12. Because the HCV-TARGET cohort revealed that age, sex, ALT levels, MELD and Child-Pugh scores were associated with short-term MELD score improvement, multivariate analysis for these factors was performed to assess factors associated with an improved MELD score of ≥3 after SOF/VEL plus RBV and was presented as odds ratio (OR) with 95% CI [9]. The evolution of eGFR from baseline to off-treatment week 12 in patients with baseline CKD stages 1 to 3 was compared using the generalized estimated equation (GEE) [24]. All statistical tests were 2-tailed, and results were statistically significant when the P value was less than 0.05.
RESULTS
Patient characteristics
Of the 137 patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis who were scheduled to receive SOF/VEL plus RBV for 12 weeks, 107 were eligible for the study after excluding 30 patients with active HCC (n=8), liver transplantation (n=1), HBV co-infection (n=11), and CKD stage 4 or 5 (n=10). A total of 102 (95.3%) and 96 (89.7%) patients completed 12 weeks of treatment and 12 weeks of off-treatment follow-up (Fig. 1). The median age was 65 years, 62 (57.9%) were males, and 98 (91.6%) were treatment-naïve. All nine treatment-experienced patients failed to respond to peginterferon plus RBV. Ninety-two (86.0%) and 15 (14.0%) patients had Child-Pugh B and Child-Pugh C cirrhosis, respectively. With regard to the MELD score, 49 (45.8%), 42 (39.3%), and 16 (15.0%) patients had baseline scores of <10, 10–14, and ≥15, respectively. Twenty-four patients (22.4%) with HCC had achieved complete ablation. Thirty-three patients (30.8%) had a history of variceal hemorrhage. Eight-six (80.4%) and 28 (26.2%) presented with ascites and hepatic encephalopathy. For HCV GT distribution, 47 (43.9%), 42 (39.3%), 10 (9.3%), and six (5.6%) patients were infected with HCV GT 1, 2, 3, and 6, and the remaining two patients (1.9%) were infected with mixed or indeterminate GTs. Fifty-three (49.5%), 37 (34.6%), and 17 (15.9%) had baseline CKD stages 1, 2, and 3, respectively (Table 1).
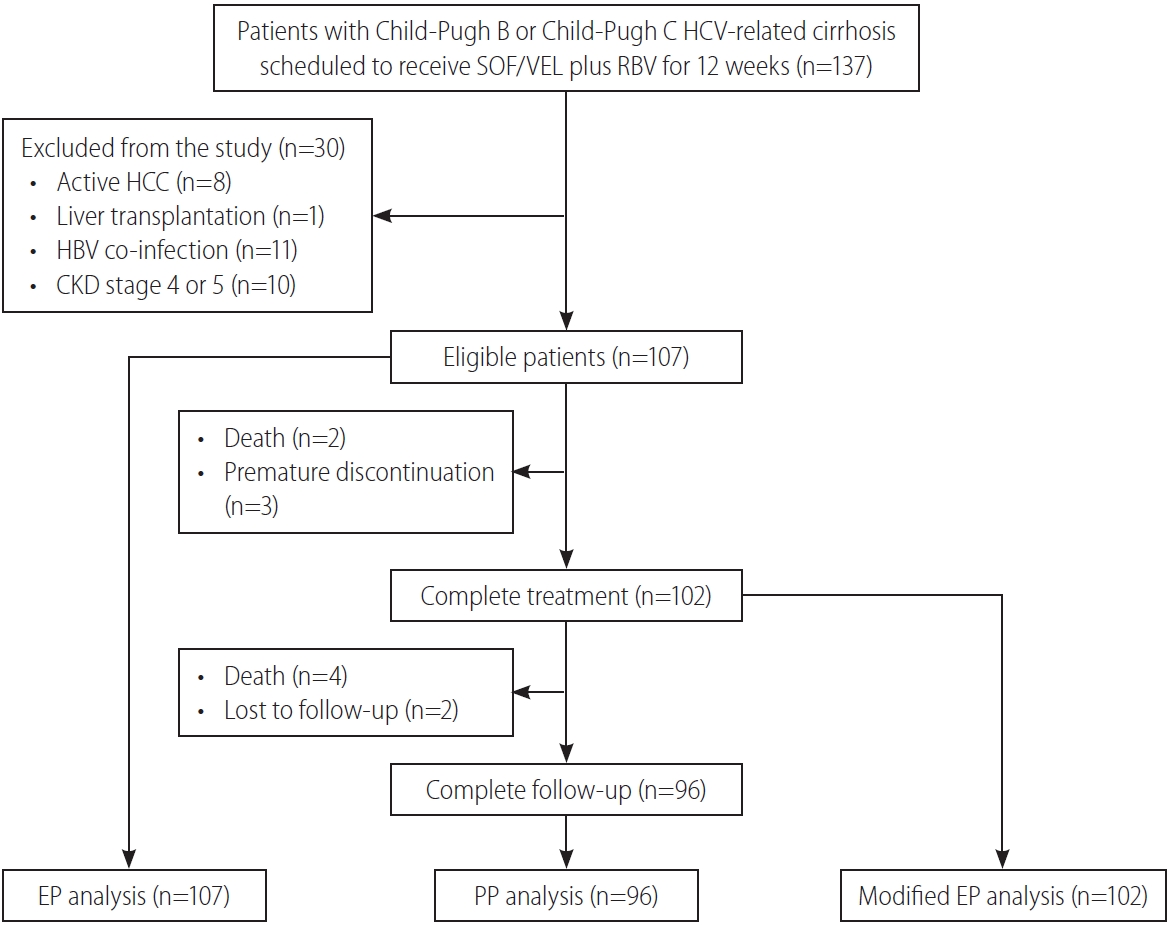
Study flow. HCV, hepatitis C virus; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; HCC, hepatocellular carcinoma; CKD, chronic kidney disease; EP, evaluable population; PP, per-protocol population.
Drug adherence
Among the 102 patients who completed SOF/VEL plus RBV for 12 weeks, 99 (97.1%) and three (2.9%) consumed ≥95% and 90–94% of the dispended SOF/VEL pills, and 33 (32.3%), 27 (26.5%), 27 (26.5%), and 15 (14.7%) consumed ≥80%, 60–79%, 40–59%, and <40% of the dispended RBV pills. Among 102 patients who completed a 12-week course of SOF/VEL plus RBV treatment, the doses of RBV were reduced to <80%, <40%, and <20% at week 4 in patients with on-treatment week 4 hemoglobin levels of 9.0–9.9 g/dL, 8.0–8.9 g/dL, and <8.0 g/dL, while the doses of RBV were not reduced in 100% and 55% patients with on-treatment hemoglobin levels of ≥12 g/dL and 11.0–11.9 g/dL at on-treatment week 4. At on-treatment week 8, the doses of RBV were reduced to <80%, <60%, and <20% in patients with on-treatment week 8 hemoglobin levels of 9.0–9.9 g/dL, 8.0–8.9 g/dL, and <8.0 g/dL, while the doses of RBV were not reduced in 94% and 50% patients with on-treatment hemoglobin levels of ≥12 g/dL and 11.0–11.9 g/dL at on-treatment week 8. In patients with on-treatment hemoglobin levels of 10.0–10.9 g/dL, the doses of RBV were reduced to 60–79% in 73% and 48% patients at on-treatment weeks 4 and 8, respectively (Supplementary Fig. 1).
Effectiveness
At on-treatment week 12, 102 patients (100%) with available data had serum HCV RNA levels < LLOQ. In the EP, modified EP and PP analyses, the SVR12 rates were 89.7% (96 of 107 patients; 95% CI, 82.5–94.2%), 94.1% (96 of 102 patients; 95% CI, 87.8–97.3%) and 100.0% (96 of 96 patients; 95% CI, 96.2–100.0%), respectively (Table 2). Among the 11 patients who failed to achieve SVR12, six, three, and two patients died, prematurely discontinued treatment and were lost to follow-up during the study period. The SVR12 rates of the subgroups in the EP analysis are shown in Figure 2.
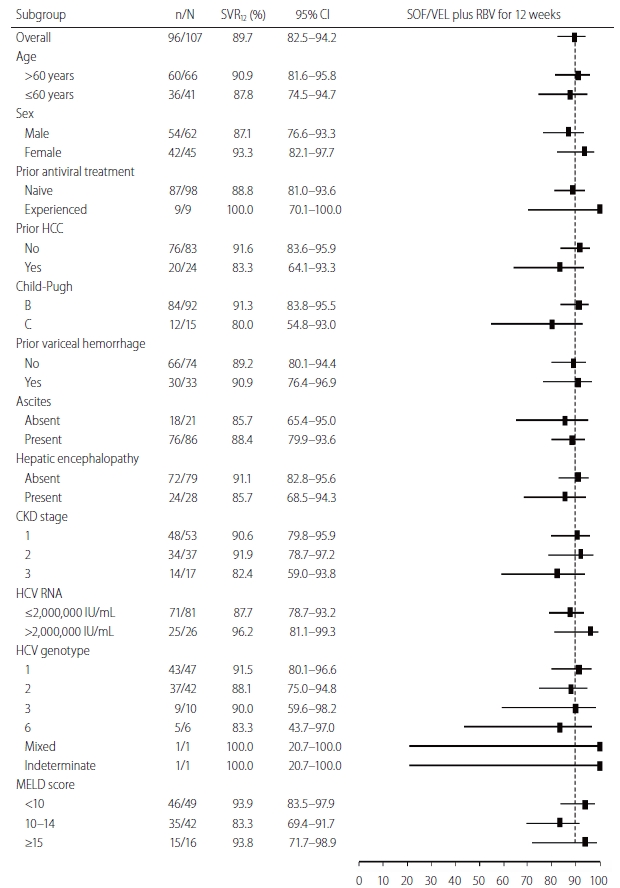
The sustained virologic response rate at off-treatment week 12 (SVR12) rates in the evaluable population analysis according to subgroup. The position of the square indicates SVR12 in each subgroup; the horizontal lines indicate 95% CIs. The dotted vertical line represents overall SVR12 rate. CI, confidence interval; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; HCC, hepatocellular carcinoma; CKD, chronic kidney disease; HCV, hepatitis C virus; MELD, model for end-stage liver disease.
Safety
A total of 98 patients (91.6%) were reported to have at least one AE. Among the 24 patients (22.4%) presenting with serious AEs, including six deaths, none were related to SOF/VEL or RBV (Table 3). Three of 92 (3.3%) and three of 15 patients (20.0%) with Child-Pugh B and Child-Pugh C cirrhosis died, and two of the six events (33.3%) were liver-related deaths. Four patients who completed 12 weeks of SOF/VEL plus RBV died during off-treatment follow-up because of hepatic encephalopathy, femoral fracture with septic shock, pneumonia, and lumbar epidural abscess. One of the three patients who discontinued treatment had treatment-related fatigue at on-treatment week 2. The other two patients discontinued treatment at on-treatment weeks 3 and 8 due to traumatic head injury and personal reasons. Common AEs with reported rates of ≥10% included fatigue (82.2%), nausea (22.4%), headache (19.6%), insomnia (16.8%), diarrhea (15.0%), dizziness (14.0%), pruritus (12.1%), and dyspnea (10.3%). Twenty-eight (26.2%) and six (5.6%) patients had on-treatment grade 2 and grade 3 anemia. Thirty-one patients (30.0%) had on-treatment grade 3 total bilirubin elevation, and 27 (87.1%) had unconjugated hyperbilirubinemia. None had an on-treatment ≥ grade 3 ALT elevation. Three (3.6%) and one (4.2%) patients had HCC occurrence and recurrence during the 24-week study period. Four patients (3.7%) experienced esophageal or gastric variceal hemorrhage during the 24-week study period, in whom one patient died (Table 3). The eGFR declines from baseline to SVR12 were more significant in patients with CKD stage 1 than in patients with CKD stage 2 (-0.42 mL/min/1.73 m2/month; 95% CI, -0.75 to -0.09; P=0.01) and in patients with CKD stage 1 than in patients with CKD stage 3 (-0.56 mL/min/1.73 m2/month; 95% CI, -0.84 to -0.29; P<0.001) (Fig. 3).
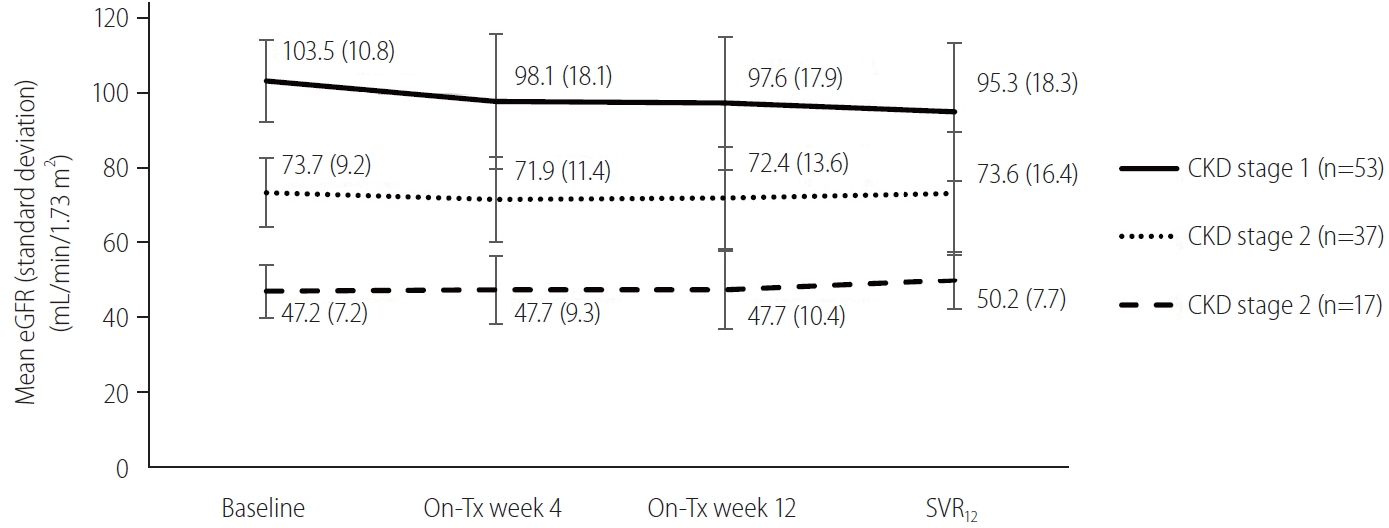
Changes of eGFR from baseline to SVR12 by GEE according to baseline CKD stage. The eGFR at different time points is shown as mean (standard deviation). The eGFR evolution from baseline to SVR12 is shown between patients with CKD stages 1 and 2 (-0.42 mL/min/1.73 m2/month; 95% CI, -0.75 to -0.09; P=0.01) and patients with CKD stages 1 and 3 (-0.56 mL/min/1.73 m2/month; 95% CI, -0.84 to -0.29; P<0.001). eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; Tx, treatment; SVR12, sustained virologic response rate at off-treatment week 12; GEE, generalized estimated equation; CI, confidence interval.
Changes of Child-Pugh and MELD scores before and after antiviral treatment
Among the 96 patients who achieved SVR12, 81 (84.4%) and three (3.1%) showed improvement and worsening in Child-Pugh scores (Fig. 4A), and 62 (64.6%) and 20 (20.8%) showed improvement and worsening MELD scores, respectively (Fig. 4B). Furthermore, 30 (31.3%) of the 96 patients who achieved SVR12 had an improved MELD score of ≥3. Multivariate analysis revealed that a baseline MELD score ≥15 (OR, 4.13; 95% CI, 1.16–14.71; P=0.02) was associated with an improved MELD score of ≥3 (Table 4). Before antiviral treatment, 86 (80.4%) and 28 (26.2%) of 107 patients presented with ascites and hepatic encephalopathy, while 34 (31.8%) and eight (7.5%) of 96 patients who achieved SVR12 presented with ascites and hepatic encephalopathy, respectively.
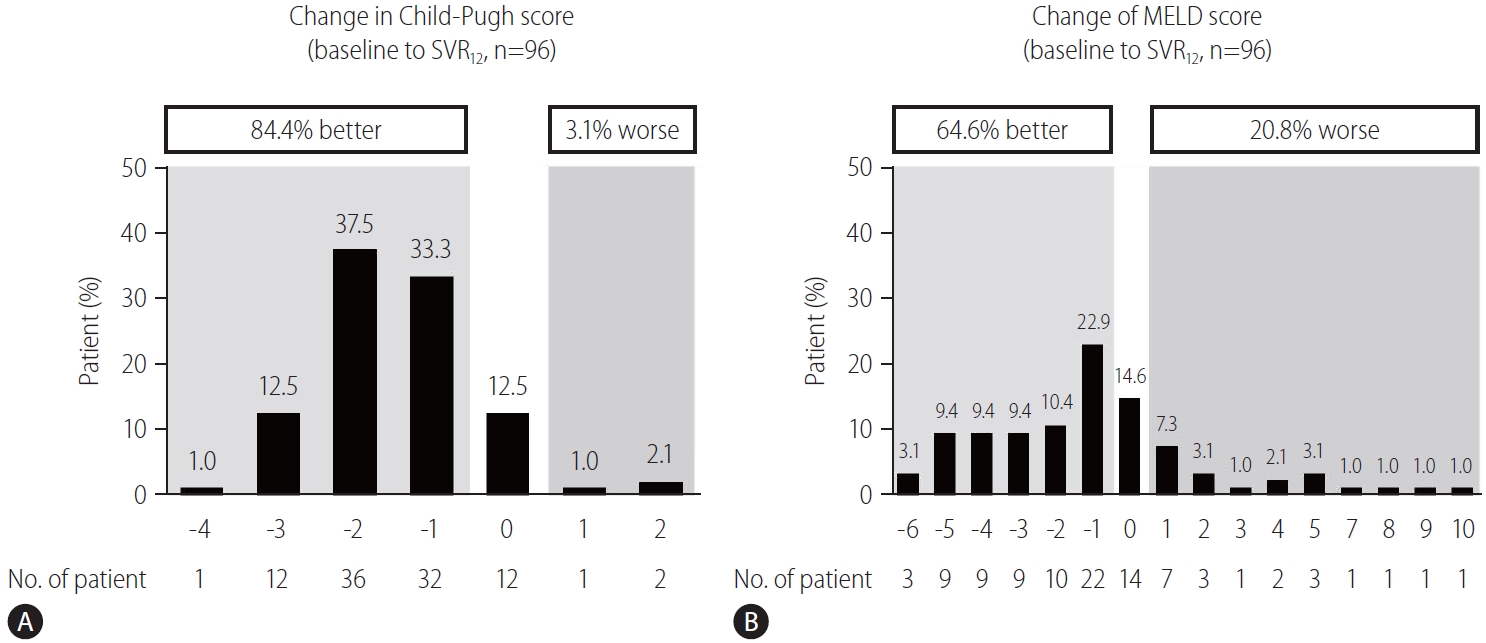
Proportion of patients achieving SVR12 with improving and worsening (A) Child-Pugh and (B) MELD scores from baseline to SVR12. SVR12, sustained virologic response rate at off-treatment week 12; MELD, model for end-stage liver disease.
DISCUSSION
Until now, SOF/VEL plus RBV is the only approved pangenotypic regimen for patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis, who are expected to have a grave prognosis if left untreated. Furthermore, this regimen is easily described by physicians with a 12-week course of treatment, limited pill burden, and low risks of significant drug-drug interactions [25]. Although several studies from Japan showed that Child-Pugh B and Child-Pugh C HCV-related cirrhotic patients who were infected with HCV GT 1b or 2 can achieve high SVR12 rates by SOF/VEL alone for 12 weeks, the guidelines and the regulatory authorities recommend SOF/VEL plus RBV for 12 weeks for this special clinical setting because the phase 3 randomized trial have demonstrated the SVR12 rate in participants receiving SOF/VEL plus RBV for 12 weeks was superior to those receiving SOF/VEL also for 12 or 24 weeks by reducing the risk of viral relapse [14,26-30]. To our knowledge, only one smallscale study from China reported 12 patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis receiving SOF/VEL plus RBV and all achieved SVR12 [16]. Our study, which included 107 patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis receiving SOF/VEL plus RBV for 12 weeks, revealed that the SVR12 rates in EP, modified EP, and PP were 89.7%, 94.1%, and 100%, respectively. The SVR12 rates of our patients with Child-Pugh B and Child-Pugh C cirrhosis receiving SOF/VEL plus RBV for 12 weeks in EP analysis were 91.3% and 80%, respectively, which were also comparable to the SVR12 rates of 94% and 70% by intention-to-treat analysis in phase 3 global trials [14,15]. Among patients who completed 12 weeks of SOF/VEL plus RBV, the SVR12 rates in Child-Pugh B and Child-Pugh C cirrhosis were 96.6% and 80.0%, respectively. In the PP analysis, the SVR12 rate in our patients receiving SOF/VEL plus RBV for 12 weeks was 100.0% irrespective of the grade of cirrhosis, which was also similar to the reported SVR12 rates to be 96.5% and 100.0% in Child-Pugh B and Child-Pugh C cirrhosis after excluding non-virologic failures in phase 3 trials [14,15]. Furthermore, the SVR12 rates in our real-world data were comparable regardless of age, sex, prior treatment history, prior HCC history, grade of hepatic decompensation, history of variceal hemorrhage, ascites, hepatic encephalopathy, renal reserve, HCV viral load, or HCV GT, which implied the excellent effectiveness of SOF/VEL plus RBV in various subgroups.
Regarding drug adherence, 97.1% of patients who completed 12 weeks of treatment consumed >95% of the dispended SOF/VEL pills. In contrast, only 32.3% of patients who completed 12 weeks of treatment consumed ≥80% of the dispended RBV pills. The excellent SOF/VEL adherence in our patients can be attributed to the easy dosing, low pill burden, and high tolerance for SOF/VEL. Although most patients needed RBV dose reduction because of on-treatment anemia or RBV-related AEs, it did not adversely affect SOF/VEL adherence. Because no patients experienced virologic failures, RBV dose reduction did not significantly reduce the SVR12 rate under high SOF/VEL adherence [14]. Four patients who had favorable on-treatment viral responses after completing SOF/VEL plus RBV for 12 weeks died of hepatic encephalopathy and infections after stopping treatment. Because the risks of liver-related and non-liver-related deaths remain high in patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis, physicians should consider liver transplantation for these vulnerable patients if liver grafts are available [31,32].
While 91.6% of our patients were reported to have at least one AE, only one patient (0.9%) discontinued treatment because of treatment-emergent AE. Moreover, all serious AEs and deaths were considered unrelated to the use of SOF/VEL or RBV. The proportions of patients with common AEs in our study were similar to those reported in clinical trials [14,15]. With regard to laboratory abnormalities, 31.8% and 30% of our patients had ≥ grade 2 anemia and grade 3 total bilirubin elevation, which were mainly attributed to RBV-induced hemolysis and unconjugated hyperbilirubinemia. The risks of HCC occurrence and recurrence in our patients were 3.6% and 4.2% respectively during a 24-week study period, which were comparable to the reported incidences from the cohort and meta-analysis studies [2,33,34]. The risk of variceal hemorrhage was 3.7% in our patients during a 24-week study period, which was comparable to the reported incidence of 12% per year [35]. Although patients with CKD stage 1 had a more significant eGFR decline than those with CKD stage 2 or 3, the differences in eGFR decline between groups were slight and no renal events were reported in our study. This finding was similar to the eGFR changes in HCV patients with compensated liver disease receiving SOF-based DAAs, implying that the renal safety of SOF/VEL remained satisfactory even in patients with Child-Pugh B and Child-Pugh C cirrhosis [36-38]. Taken together, the safety profiles in our study confirmed the excellent tolerance of SOF/VEL plus RBV in Child-Pugh B and Child-Pugh C HCV-related cirrhosis.
Among patients achieving SVR12, 84.4% and 64.6% had improved Child-Pugh and MELD scores, and the proportions of patients with ascites and hepatic encephalopathy were significantly reduced, indicating that successful viral eradication may improve the health-related outcomes by halting virus-related liver damage [7,8]. The beneficial effect of DAA-induced SVR12 was evident by a significant decrease of patients in the USA on liver transplant waiting list in the era of DAA [39]. Of particular note, 31.3% of our patients achieving SVR12 had an improved MELD score of ≥3, which was in accordance with the HCV-TARGET cohort showing that 24% of patients with advanced/decompensated cirrhosis had an improved MELD score of ≥3 during short-term post-SVR follow-up [9]. Furthermore, our patients with a baseline MELD score ≥15 were associated with such MELD score improvement [9,14]. However, the short-term MELD “purgatory” may not necessarily confirm long-term MELD score improvement, and ongoing surveillance is still required to ensure patient survival [9]. On the contrary, patients who had worsening MELD score or who had a MELD score ≥18 despite achieving SVR12 should be referred for liver transplantation based on the anticipated high risk of death [31].
In addition to its excellent effectiveness and safety, SOF/VEL plus RBV for 12 weeks has also been shown to be cost-effective and can improve patient-reported outcomes in patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis [40,41]. These beneficial effects provide robust evidence for timely therapeutic intervention in this difficult-to-treat population.
Our study had several limitations. First, the number of patients in our study was relatively small. Therefore, our study may have reporting biases because most patients had Child-Pugh B cirrhosis and had HCV GT 1b and GT2 infection, who were considered easier to treat compared to those with Child-Pugh C cirrhosis or those with HCV GT 1a or GT 3 infection. Furthermore, this study recruited patients at referral centers, and the effectiveness and safety of SOF/VEL plus RBV for patients receiving treatment at regional hospitals or clinics need confirmation. Second, we did not include patients with CKD stage 4 or 5 because we have demonstrated that the SVR12 rates of SOF/VEL with low-dose RBV were 95% and 100% in the EP and PP analyses [42]. Third, this retrospective study lacked standardized report forms for AEs and were therefore prone to reporting biases. Fourth, we did not assess the long-term evolution of Child-Pugh score, MELD score, eGFR, or other clinical events beyond the SVR12.
In summary, our real-world study shows that SOF/VEL plus RBV for 12 weeks is efficacious and well-tolerated in patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis. Most patients have improved Child-Pugh and MELD scores following SVR12. However, close monitoring of patients achieving SVR12 is needed to ensure long-term survival.
Acknowledgements
The authors thank Hui-Ju Lin and Pin-Chin Huang for clinical data management; the 7th Core Lab of National Taiwan University Hospital and the 1st Common Laboratory of National Taiwan University Hospital, Yun-Lin Branch for instrumental and technical support.
This study was supported by Ministry of Science and Technology, Taiwan (107-2314-B-002-038-MY2).
Notes
Authors’ contributions
Conception and design: CH Liu, JH Kao; Analysis and interpretation of data: CH Liu; Drafting of the article: CH Liu, JH Kao; Critical revision of the article for important intellectual content: CH Liu, CY Chen, WC Su, CJ Liu, CC Lo, KJ Huang, JJ Chen, KC Tseng, CY Chang, CY Peng, YL Shih, CS Huang, WY Kao, SS Yang, MC Tsai, JH Wu, PY Chen, PY Su, JJ Huang, YJ Fang, PL Lee, CW Tseng, FJ Lee, HC Lai, TY Hsieh, CC Chang, CH Chang, YJ Huang, JH Kao; Final approval of the article: CH Liu, CY Chen, WC Su, CJ Liu, CC Lo, KJ Huang, JJ Chen, KC Tseng, CY Chang, CY Peng, YL Shih, CS Huang, WY Kao, SS Yang, MC Tsai, JH Wu, PY Chen, PY Su, JJ Huang, YJ Fang, PL Lee, CW Tseng, FJ Lee, HC Lai, TY Hsieh, CC Chang, CH Chang, YJ Huang, JH Kao; Provision of study materials or patients: CH Liu, CY Chen, WC Su, CJ Liu, CC Lo, KJ Huang, JJ Chen, KC Tseng, CY Chang, CY Peng, YL Shih, CS Huang, WY Kao, SS Yang, MC Tsai, JH Wu, PY Chen, PY Su, JJ Huang, YJ Fang, PL Lee, CW Tseng, FJ Lee, HC Lai, TY Hsieh, CC Chang, CH Chang, YJ Huang, JH Kao; Statistical expertise: CH Liu; Administrative, technical, or logistic support: CH Liu, JH Kao; Collection and assembly of data: CH Liu.
Conflicts of Interest
Chen-Hua Liu: advisory board for Abbvie, Gilead Sciences, Merck Sharp & Dohme; speaker’s bureau for Abbott, Abbvie, Gilead Sciences, Merck Sharp & Dohme; research grant from Abbvie, Gilead Science, Merck Sharp & Dohme. Sheng-Shun Yang: advisory board for Abbvie, Roche, Ipsen; speaker’s bureau for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Ipsen, Merck Sharp & Dohme. Jia- Horng Kao: advisory board for Abbott, Abbvie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche; speaker’s bureau for Abbott, Abbvie, Bayer, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche. All the other authors declare no competing interests.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
RBV dose adjustment according to hemoglobin levels at on-treatment weeks 4 and 8. RBV, ribavirin.
Abbreviations
AE
adverse event
ALT
alanine transaminase
AST
aspartate transaminase
CI
confidence interval
CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
CKD
chronic kidney disease
CTCAE
Common Terminology Criteria for Adverse Events
DAA
direct-acting antiviral
eGFR
estimated glomerular filtration rate
EP
evaluable population
GEE
generalized estimated equation
GT
genotype
HBsAg
hepatitis B virus surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
HIV
human immunodeficiency virus
IFN
interferon
INR
international normalized ratio
IQR
interquartile range
LLOQ
lower limit of quantification
MELD
model for end-stage liver disease
OR
odds ratio
PP
per-protocol population
RBV
ribavirin
SOF
sofosbuvir
SVR12
sustained virologic response rate at off-treatment week 12
ULN
upper limit of normal
VEL
velpatasvir
References
Article information Continued
Notes
Study Highlights
• The SVR12 rates in our real-world patients with Child-Pugh B and Child-Pugh C HCV-related cirrhosis receiving SOF/VEL plus RBV for 12 weeks were 89.7%, 94.1%, and 100% in the EP, modified EP, and per-protocol population. The tolerance was also excellent.
• Most patents who achieved SVR12 had improved Child-Pugh and MELD scores. The renal safety was excellent irrespective of baseline renal reserve.
• Our real-world study provides robust evidence to practitioners that SOF/VEL plus RBV for 12 weeks is efficacious and well-tolerated for Child-Pugh B and Child-Pugh C HCV-related cirrhosis.