Systematic review with meta-analysis: Non-alcoholic fatty liver disease and the association with pregnancy outcomes
Article information
See the commentary-article "Nonalcoholic fatty liver disease-based risk prediction of adverse pregnancy outcomes: Ready for prime time?" on page 47.
Abstract
Background/Aims
Maternal and fetal outcomes in pregnant patients with Non-alcoholic fatty liver disease (NAFLD) have been largely unexplored. To determine the level of evidence associated with maternal and fetal outcomes in pregnant women with NAFLD.
Methods
We conducted a comprehensive literature search. The studies included pregnant patients with a previous, current or subsequent diagnosis of NAFLD. We used a random-effects model using odds ratios (OR) with 95% confidence intervals (CI).
Results
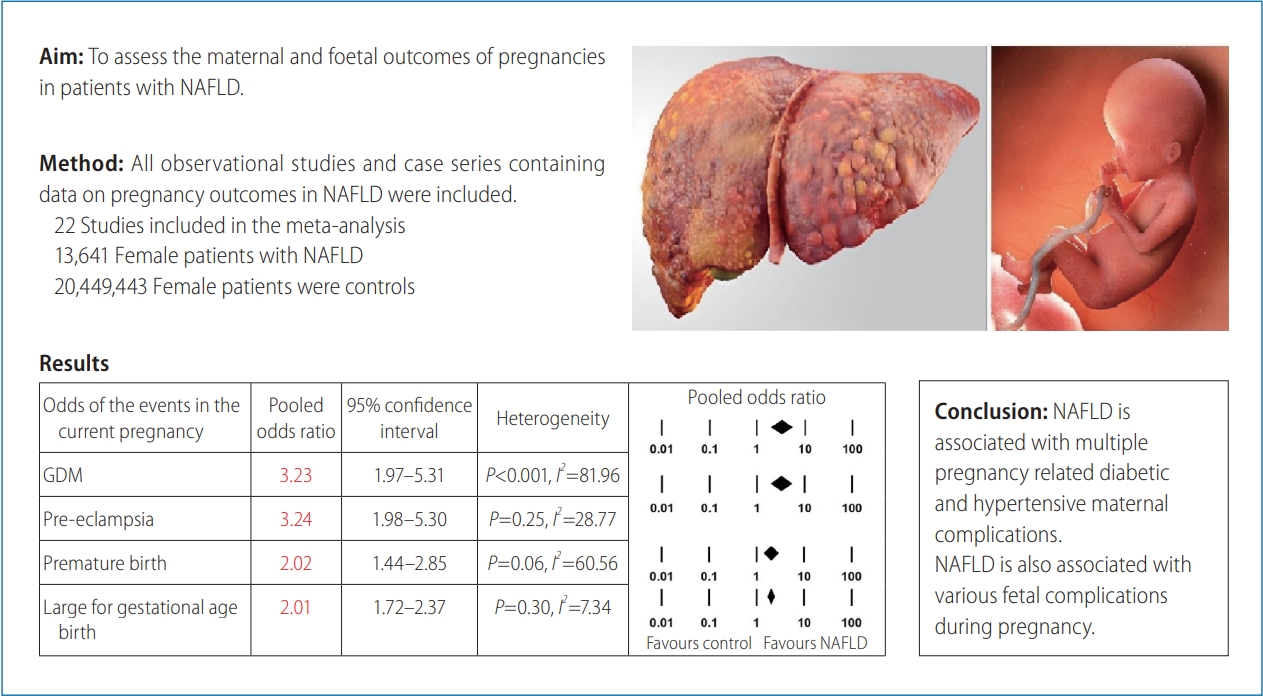
Twenty-two studies, with 13,641 female NAFLD patients were reviewed. The results highlight that NAFLD patients had a statistically significant increased likelihood of baseline diabetes mellitus (OR, 6.00; 95% CI, 2.21–16.31; P<0.001; n=7), baseline Hypertension (OR, 3.75; 95% CI, 2.13–6.59; P<0.001; n=4), gestational hypertension (OR, 1.83; 95% CI, 1.03–3.26; P=0.041; n=2), and pre-eclampsia (OR, 2.43; 95% CI, 1.46–4.04; P=0.001; n=3). The odds for a past and current history of gestational diabetes mellitus were OR, 3.78; 95% CI, 2.21–6.44; P<0.001; n=5 and OR, 3.23; 95% CI, 1.97– 5.31; P<0.001; n=6, respectively. As for fetal outcomes, pregnant NAFLD patients were significantly more likely to have a premature birth (OR, 2.02; 95% CI, 1.44–2.85; P<0.001; n=4), large for gestational age birth (OR, 2.01; 95% CI, 1.72–2.37; P<0.001; n=2) or a history of prior miscarriage or abortion (OR, 1.15; 95% CI, 1.02–1.30; P=0.02; n=2). Egger’s regression revealed no evidence of publication bias (P>0.05).
Conclusions
This meta-analysis provides pooled evidence that NAFLD is associated with a substantial increase in maternal diabetic and hypertensive complications and multiple adverse fetal outcomes. This data is important for clinicians managing these patients before, during and after pregnancy.
Graphical Abstract
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common overall cause of chronic liver disease globally, with a region-specific prevalence ranging from 13.5% to 31.8% [1]. It is a disease characterized by hepatic steatosis on imaging or biopsy, insulin resistance and increased triglyceride synthesis [2]. Whilst not being an explicit diagnostic requirement for the metabolic syndrome, NAFLD is increasingly considered a central component of it and its hepatic manifestation. NAFLD may advance to the severe form non-alcoholic steatohepatitis (NASH), a step towards the progression to cirrhosis. Histologically, these NAFLD forms are distinct; while the former has steatosis with lobular or portal inflammation, the latter is characterized by hepatocyte ballooning and hepatic lobular inflammation with or without fibrosis [3]. Although NAFLD’s overall comorbidity center on cardiovascular and metabolic/endocrine complications, it is in NASH where the hepatic sequelae from chronic liver disease state and risk of hepatocellular carcinoma occurrence are often seen.
Pregnancy and NAFLD are both insulin-resistant states [2], and their sequelae on maternal and fetal outcomes is of great interest. The relationship between gestational diabetes mellitus (GDM) and NAFLD in pregnancy appears to be bidirectional. It is established in the literature that GDM is associated with significant odds of developing intra-partum and post-partum NAFLD [4,5]. A notable proportion of studies assessed NAFLD from the GDM perspective [5-9]. The adverse effects of GDM and obesity on pregnancy have been extensively studied, yet it is only in recent years that NAFLD during pregnancy and its maternal and fetal effects has been the primary focus of various observational studies. To the best of our knowledge, no meta-analysis or systematic review has addressed this question.
The prevalence of NAFLD in women of childbearing age is estimated to be lower than that of the general adult population at 10% [10], likely due to younger age, and the overall protective effects of estrogen compared to male subjects. Pregnancy is a period of rapid weight change and estrogen fluctuations, which have a complex interplay and the causative effects of these factors towards NAFLD development or progression are unclear.
The aim of this meta-analysis to determine the level of evidence associated with both maternal and fetal outcomes in pregnant women with NAFLD. Understanding these risk factors would provide strong evidence based on which we can support NAFLD patients in their reproductive years. It will also enhance primary prevention strategies by identifying risk factors which herald the future development of NAFLD.
MATERIALS AND METHODS
Study protocol
We followed the Meta-analysis of Observational Studies in Epidemiology guidelines [11]. A systematic search of the databases MEDLINE (Ovid), PubMed, Google Scholar, EMBASE and Web of Science was performed up to August 2021, to identify relevant articles. PubMed search items included: non-alcoholic fatty liver disease OR non-alcoholic fatty liver OR non-alcoholic steatohepatitis AND pregnancy OR gestation OR birth OR obstetric OR menarche OR menopause OR parity OR gravidity. Furthermore, EMBASE, MEDLINE and Cochrane Library searches utilized MeSH terms. The reference lists of relevant articles were also searched for appropriate studies. The search included an assessment of unpublished literature. All the studies included focused on NAFLD during a pregnancy [12-19], NAFLD as an associated outcome in pregnancy; often to GDM [5-9,20,21] or evaluated NAFLD in women assessing for co-variates such as hormonal and lifestyle factors [22-28]. Pregnancy related outcomes were only extracted from the first two groups.
Study selection
We included studies that met the following inclusion criteria: 1) radiological criteria (ultrasonography or computer tomography) or any of histological (liver biopsy) findings consistent with NAFLD in the absence of other chronic liver disease; 2) female of any age group with at least one prior or current pregnancy. Eligible study designs consisted of observational studies and case series with full published articles or grey literature sources in the English language. The primary outcome was comparing NAFLD maternal absolute risk of diabetic and hypertensive complications and clinically significant neonatal adverse outcomes to the non-NAFLD pregnancies. Secondary outcomes included comparing the demographic associations of NAFLD including race, parity, and obesity to the general pregnancy population. Exclusion criteria were studies with pediatric population, case reports and review articles. Studies with non-radiological or biopsy-based diagnoses of NAFLD such as using the hepatic steatosis index were excluded.
Data extraction
The literature search was conducted from June 2020 to August 2021. The data extraction was performed by two investigator authors (HEJ, GDE) using a standardized data extraction form, collecting information on the following maternal outcomes: pregnancy related outcomes of miscarriage, abortion, smoking status, diabetes mellitus (DM), GDM, gestational hypertension (GHTN), pre-eclampsia and history of these conditions. Both authors reached consensus on the included studies. Furthermore, basic demographics such as age, body mass index (BMI), weight and race were collected. Fetal outcomes were neonatal mortality, premature birth, congenital defects, large for gestational age birth (LGA) and mode of delivery (vaginal vs. low section caesarean section [LSCS]). Maternal and fetal mortality was collected. All data analysis is based on the number of patients rather than the number of pregnancies. All available data relating to maternal or neonatal outcome measures in NAFLD pregnancies were coded. Additional information was also collected on the publication year, study design, number of cases, number of controls, total sample size, temporal direction, and continent. We contacted five authors (of five studies) requesting further information on the total number of pregnancies though we did not receive a reply.
Quality assessment
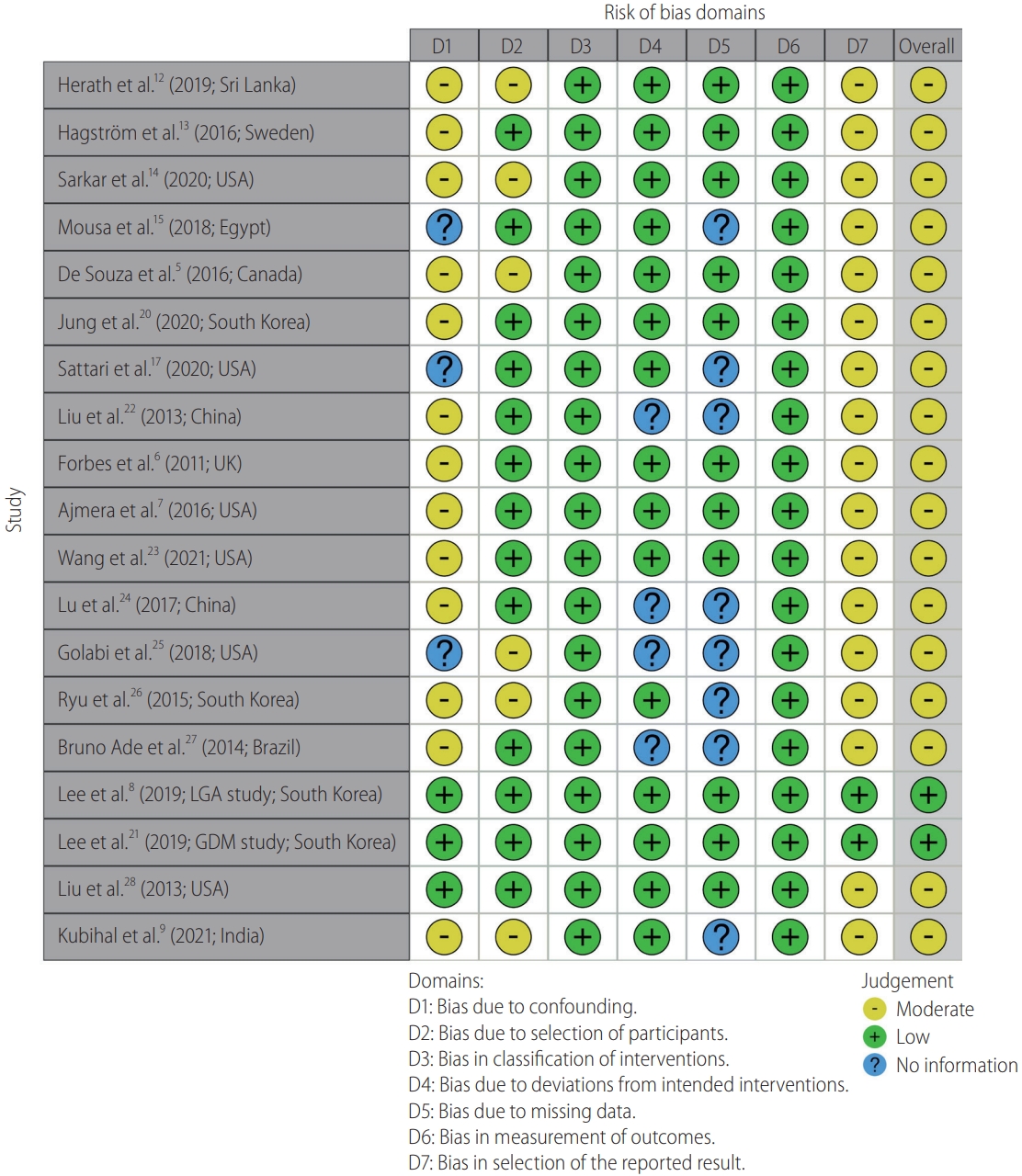
Quality assessment of the included observational studies was performed using the Newcastle-Ottawa Scale [29] and case series utilized the MOGA scale (Table 1) [30]. These scales assess each article on selection of the study groups, confounding, and comparability of the groups and ascertainment of the outcome of interest. The risk of bias was summarized using the ROBINS-I risk of bias tool for non-randomized controlled studies (Fig. 1) [31]. Finally, the quality of the evidence for the outcomes was rated using the GRADE system (Supplementary Table 1) [32]. Agreement regarding the marking of each study was reached by consensus.
Statistical analysis
We used a random-effects model for this analysis, using prevalence rates (event rates) and odds ratio (OR) with 95% confidence interval (CI) [33]. A random effect model was used due to varying effect sizes across the included studies. We tested heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies that is due to heterogeneity. I2 values of 50% or above corresponded to a substantial degree of heterogeneity [34]. We assessed publication bias using the Egger’s regression model only if there were greater than ten studies [35]. Differences in continuous variables were measured using difference in means. All analyses were performed with comprehensive meta-analysis (version 3.0; Biostat, Englewood, NJ, USA). Pooled odds ratios were based on two-armed observational studies, whilst prevalence data were obtained from all included studies.
RESULTS
Search strategy
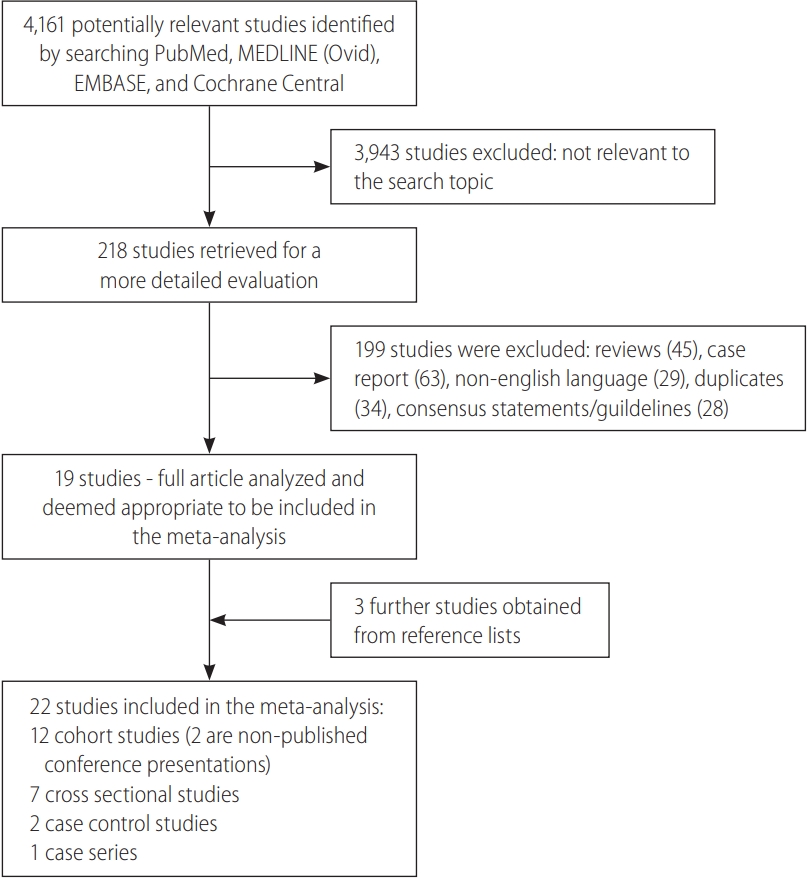
A total of 4,161 publications were searched. Of these 3,943 publications were eliminated based on title and abstract search. The remaining 218 publications were independently assessed: 19 fulfilled inclusion criteria. Of those remaining 19 articles which met inclusion criteria, a detailed search in their reference lists yielded three further studies which met the study’s criteria for inclusion. In total, 22 articles [5-9,12-28] were included in this meta-analysis (Fig. 2).
Study characteristics
The 22 studies included a total of 13,641 female patients with NAFLD. There were 12 studies performed intrapartum, four studies in pre-menopausal women post-partum and six studies on post-menopausal women with a breakdown of 6,559, 1,603, and 5,479 patients, respectively. The individual study characteristics are shown in Supplementary Table 2. The control population consisted of 20,449,443 women. Twelve studies were cohort studies, seven were cross-sectional studies, two were case-control studies, and one was case series. Of the 15 non-cross-sectional studies, 10 were prospective and five were retrospective. Participants were enrolled between years 1992 and 2019. The studies were conducted in different databases and timeframes, hence it is extremely unlikely for the same patient to be enrolled in multiple studies, with the exception of the two studies by Lee et al. [8,21]. From these two studies, patients were counted once towards the total number of patients in the analysis, and they assessed mutually exclusive outcomes. The follow-up period for studies conducted during a pregnancy ranged from the first antenatal visit in the first trimester to 12 weeks post-natal visit. Sixteen studies assessed for biochemical parameters of liver function. Of the total studies, 20 were full publications whilst two studies were conference abstracts.
Maternal outcomes
The prevalence rates for various maternal outcomes are shown in (Table 2, Supplementary Table 1). Pregnant women with NAFLD had six times the odds for baseline DM (OR, 6.00; 95% CI, 2.21– 16.31; P<0.001; n=7) compared to the control group with a considerable heterogeneity I2=98.92, P<0.001. They were also notably more likely to have a current GDM diagnosis (OR, 3.23; 95% CI, 1.97–5.31; P<0.001; n=6) and remarkably more likely to have a previous history of GDM (OR, 3.78; 95% CI, 2.21–6.44; P<0.001; n=5) with a substantial degree of heterogeneity; I2=84.59, P<0.001 and I2=64.87, P=0.02, respectively. Forest plots for the outcomes of current GDM and for pre-eclampsia are shown in Figure 3.
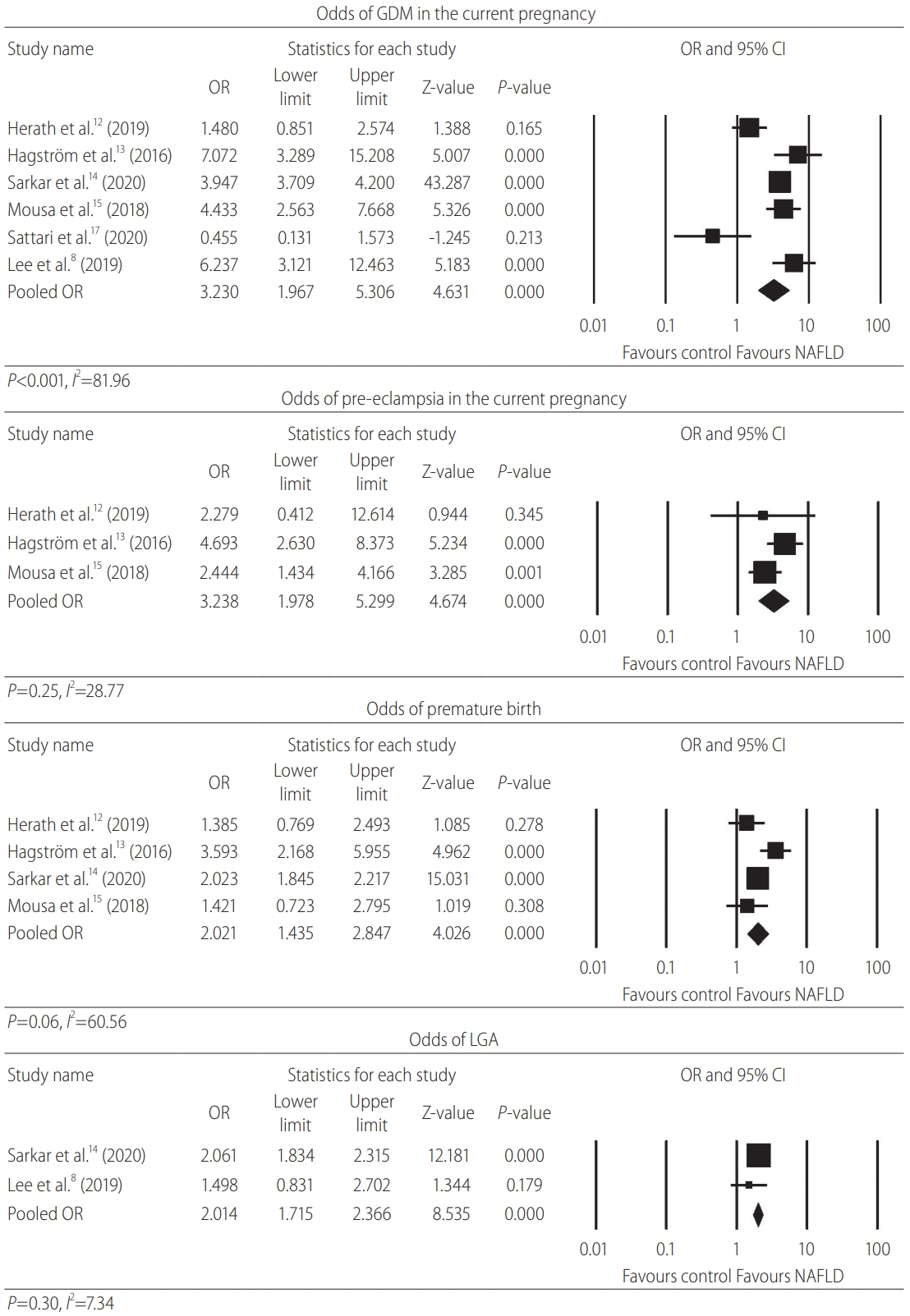
Forest plot for current GDM, pre-eclampsia, premature, and LGA births. GDM, gestational diabetes mellitus; OR, odds ratio; CI, confidence interval; NAFLD, non-alcoholic fatty liver disease; LGA, large for gestational age birth.
Similar to the diabetic parameters above, the odds for hypertensive conditions were significantly elevated. Females with NAFLD were significantly more likely to have pre-pregnancy hypertension (OR, 3.75; 95% CI, 2.13–6.59; P<0.001; n=4) compared to the control group with a substantial degree of heterogeneity I2=85.34, P<0.001. They were also markedly more likely to have a current GHTN diagnosis (OR, 1.83; 95% CI, 1.03–3.26; P=0.041; n=2) compared to controls with a moderate degree of heterogeneity I2=43.81, P=0.18. In addition, pre-eclampsia was strongly associated with NAFLD (OR, 3.24; 95% CI, 2.21–4.75; P<0.001; n=3) with a low degree of heterogeneity I2=28.77, P=0.25. The odds for the composite outcome of pre-eclampsia, eclampsia or HELLP (haemolysis, elevated liver enzymes, low platelet count) syndrome in NAFLD pregnancies were almost four-times higher (OR, 3.91; 95% CI, 2.71–5.64; P<0.001; n=4) compared to the control population with a substantial degree of heterogeneity I2=54.30, P=0.09.
NAFLD related pregnancies yielded over two-times the odds for post-partum haemorrhage (OR, 2.03; 95% CI; 1.83–2.27; P<0.001; n=1) and almost 18-times the odds for maternal death (OR, 17.80; 95% CI, 7.39–42.88; P<0.001; n=1), although these parameters were based on a single study. There was no difference in NAFLD females or controls giving birth via vaginal delivery (OR, 1.02; 95% CI, 0.65–1.58; P=0.95; n=1) which was not statistically significant. However, NAFLD females were significantly more likely to give birth via LSCS (OR, 2.05; 95% CI, 1.19–3.53; P=0.01; n=3) compared to controls with a substantial level of heterogeneity I2=89.85, P<0.001.
Fetal outcomes
The prevalence rates for various fetal outcomes are shown in Table 2 and Supplementary Table 1. Females with NAFLD had a significant doubling of the odds of premature birth (OR, 2.02; 95% CI, 1.44–2.85; P<0.001; n=4) and LGA birth (OR, 2.01; 95% CI, 1.72–2.37; P<0.001; n=2) with a substantial (I2=60.56, P=0.06), and a low (I2=7.34, P=0.30) degree of heterogeneity respectively. NAFLD pregnancies had a significant association with a history of a previous miscarriage or termination (OR, 1.15; 95% CI, 1.02–1.30; P=0.02; n=2) with low evidence of heterogeneity, I2<0.001, P=0.64. The odds of low birth weight were higher but did not reach statistical significance (OR, 1.55; 95% CI, 0.45–5.33; P=0.49; n=2) with a substantial degree of heterogeneity, I2=88.22, P=0.004. The odds for fetal death in NAFLD pregnancies was higher but did not reach statistical significance (OR, 1.15; 95% CI, 0.86–1.55; P=0.34; n=1). The odds for intrauterine growth restriction were lower without reaching statistical significance (OR, 0.88; 95% CI, 0.44–1.77; P=0.73; n=2) with a substantial degree of heterogeneity, I2=78.26, P=0.03. Forest plots for the outcomes of premature and LGA birth are shown in Figure 3.
Baseline characteristics
Parity
Women with a NAFLD pregnancy were significantly less likely to be in their first pregnancy compared to controls (primigravida) (OR, 0.64; 95% CI, 0.47–0.85; P=0.003; n=2) with low evidence of heterogeneity, I2<0.001, P=0.96. The odds for a second pregnancy were not statistically different between both groups (OR, 1.17; 95% CI, 0.88–1.56; P=0.29; n=2) with low evidence of heterogeneity, I2<0.001, P=0.35. NAFLD pregnancies were significantly more likely to be a woman’s third pregnancy or higher (multigravida >3) (OR, 1.52; 95% CI, 1.1–2.1; P=0.01; n=2) with low evidence of heterogeneity, I2<0.001, P=0.43. However, the increased odds for a higher parity with NAFLD did not continue to hold true for a fourth pregnancy or higher using two different studies (multigravida >4) (OR, 1.04; 95% CI, 0.78–1.38, P=0.81; n=2) with a substantial degree of heterogeneity, I2=74.32, P=0.05.
Weight
Females with NAFLD have over eight-times the odds for concomitant obesity (OR, 8.44; 95% CI, 8.00–8.89; P<0.001; n=2) with low evidence of heterogeneity, I2<0.001, P=0.36. The odds of being overweight in a NAFLD pregnancy compared to controls was not statistically different (OR, 0.94; 95% CI, 0.59–1.51; P=0.81; n=1) from a single study. However, the odds of overweight or obesity (BMI >25) were significantly higher in the NAFLD group (OR, 4.82; 95% CI, 2.74–8.48; n=2) with substantial evidence of heterogeneity, I2=66.23, P=0.09. Obesity and overweight were defined as a BMI of >30 and 25–30, respectively. During pregnancy, NAFLD patients had a significantly higher BMI compared to the control group (standard difference in means: +1.34; 95% CI, 0.30–2.37; P=0.01; n=3).
Ethnicity
A Hispanic background was significantly associated with a NAFLD pregnancy compared to controls (OR, 2.38; 95% CI, 2.25–2.51; P<0.001; n=3) with low evidence of heterogeneity, I2<0.001, P=0.95. The odds for patients of a Caucasian background in a NAFLD pregnancy is slightly lower (OR, 0.97; 95% CI, 0.64–1.47; P=0.89; n=4) with a considerable degree of heterogeneity, I2=94.62, P<0.001. The likelihood of a NAFLD pregnancy being associated with an African-American background compared to the control group was lower without reaching statistical significance (OR, 0.69; 95% CI, 0.40–1.20; P=0.19; n=4) with a considerable degree of heterogeneity, I2=94.05, P<0.001.
Age
A pooled analysis into the difference in maternal age at delivery between the NAFLD and the control group pregnancies showed no significant difference (standard difference in means: +0.132; 95% CI, -0.006 to 0.271; P=0.06; n=4).
Smoking
NAFLD pregnant women were more likely to be current smokers (during pregnancy) compared to controls without reaching statistical significance (OR, 1.22; 95% CI, 0.91–1.65; P=0.18; n=3) with a moderate degree of heterogeneity, I2=33.09, P=0.22. They were also more likely to be smokers into the future (OR, 1.38; 95% CI, 0.73–2.64; P=0.32; n=2) based on two studies assessing NAFLD in post-menopausal women and prior pregnancy outcomes, with a substantial degree of heterogeneity, I2=65.04, P=0.09.
Autoimmune disease
NAFLD pregnant women had a significantly raised odds for having concomitant autoimmune thyroid disease (AITD) compared to controls (OR, 9.89; 95% CI, 3.01–32.46; P<0.001; n=1).
Quality assessment
A quality assessment was conducted on all included studies (Table 1). Overall, the observational studies score was of moderate to high quality.
DISCUSSION
This meta-analysis provides collective evidence that NAFLD is independently associated with a significant increase in maternal conditions of baseline HTN and DM, obesity, GDM, history of GDM, pre-eclampsia, and composite outcomes of hypertensive complications of pregnancy. NAFLD pregnant patients show a significantly increased fetal risks of preterm birth, LGA birth as well as a history of abortion or miscarriage. Importantly, the data also suggests that the odds of NAFLD are substantially higher for women of Hispanic ethnicity and those with increased parity. This data is important for clinicians managing these patients before, during and after pregnancy. It is also important for pregnant NAFLD patients to be educated about the long-term sequelae of this metabolic condition. There should be an awareness that a prior or current episode of GDM indicates a high index of suspicion of NAFLD.
The higher BMI (>30 as opposed to >25) and the more established diabetic state (DM as opposed to GDM) groups have shown a stronger association with NAFLD in pregnancy. In our analysis, whilst there were moderately elevated odds for GDM, past Hx of GDM, and overweight or obesity (BMI >25; OR, 3.2–4.8), the association was notably higher for obesity (BMI >30) or baseline diabetes mellitus (OR, 6.0–8.4). NAFLD shares the attribute of peripheral insulin resistance with other manifestations of the metabolic syndrome such as obesity and type 2 DM [36], and hence a stronger association with disease states that have a higher degree of insulin resistance would be expected.
There is a higher prevalence of obesity and insulin resistance in patients from a Hispanic and African-American backgrounds, compared to the general USA population [37]. Whilst patients of Hispanic background experience a higher rate of visceral adiposity, patients of an African-American background have a favourable visceral adiposity, lipid profile and lower NAFLD prevalence compared to the populace [37,38]. In our analysis limited to female patients, similar findings were demonstrated whereby patients of a Hispanic ethnicity had a significantly higher odds with NAFLD compared with patients of an African-American background having an inversely proportional relationship to NAFLD which was not significant. Similarly, a Swedish study has observed a significant association between being Iraqi-born and NAFLD (based on fatty liver index) compared to the Swedish-born citizens [39]. This indicates the potential for genetics and lifestyle choices as important factors in NAFLD, and that in certain backgrounds the significance of NAFLD and its implications on pregnancy outcomes may be a more prevalent issue.
In our analysis, pooled data showed a significant association between NAFLD and increasing parity (OR, 0.64, 1.17, and 1.52 for 1st, 2nd, and 3rd or more pregnancies, respectively, n=2). One of the two studies in the analysis adjusted parity for maternal age and obesity status and achieved the same significant association [11]. The link between NAFLD and ageing is apparent, as the former is a manifestation of a chronic progressive metabolic disease. It is likely that the independently increased risk of NAFLD with increasing parity is related to a combination of hormonal and lifestyle factors. There is evidence that women with higher parity have lower measured follicular estradiol levels compared to nulliparous women [40]. Estrogen acts to decrease appetite and food intake by various mechanisms and hence a lower estrogen in parous women could contribute to weight gain and NAFLD. This interesting finding of a potential link between parity and NAFLD in the absence of obesity perhaps points to other hormonal factors or the aforementioned lifestyle factors yet to be explored.
The remarkable link between NAFLD and GDM and/or baseline DM has multiple clinical implications. Firstly, all NAFLD patients warrant the highest tier risk for screening for GDM early to avoid the gestational morbidity of DM by detecting and managing it appropriately. Secondly, given GDM is a well-established risk factor for future development of type 2 DM [41], NAFLD is an early warning marker for those higher risk women at risk for diabetes mellitus to be screened early after pregnancy. Finally, NAFLD may continue post-partum and can be influenced by lifestyle modifications and is a marker to potentially monitor for metabolic disease. Current evidence indicates that NAFLD increases the risk of subsequent GDM in the same pregnancy [5,8], yet, the bidirectional nature of the relationship between these two conditions and the shared mechanisms suggests that targeting the former will likely reduce the future occurrence of the latter. Further long-term follow-up studies are warranted to establish this relationship.
The mechanism by which NAFLD causes impaired glucose tolerance is likely due to a shared pro-inflammatory adipokine and hepatokine-mediated response [10]. Some of these have been measured in the included studies [8] and showed trends such as low adiponectin and high selenoprotein which correlated with NAFLD in the first trimester and subsequent GDM in the second trimester. It is possible that NAFLD and GDM are distinct metabolic diseases that share a common metabolic dysfunction such as insulin resistance [4,13]. The significantly higher odds for obesity in the NAFLD cohort can independently account for pre-eclampsia and GDM as a well-recognized risk factor [42,43]. Lee et al. [8] has demonstrated that developing GDM in the second trimester in women with NAFLD (diagnosed in the first trimester) was significant at OR, 2.50 (P<0.05); despite adjusting for age, abdominal obesity, insulin resistance (based on Homeostatic Model Assessment for Insulin Resistance) and various adipokines and hepatokines. This highlights the presence of other mediators implicated in NAFLD or GDM and other unknown mechanisms. Other potential factors include the glycoprotein ‘sex-hormone binding globulin’ which has been shown to be inversely proportional with the prevalence of NAFLD in middle aged women [44]. The roles these mediators play during pregnancy is not well understood. During pregnancy, the baseline insulin resistance state present in NAFLD is further challenged by the secretion of placental lactogen hormone as well as other placental hormones that could worsen systemic insulin resistance and subsequently lead to the elevation of maternal blood glucose levels to facilitate the supply of energetic substrates to the fetus [45]. The mechanism of fetal macrosomia is hypothesized by Pederson’s hypothesis [46] of maternal hyperglycemia leading to fetal hyperinsulinemia, increased uptake of glucose and increased fetal adipose tissue. Fetal prematurity is a multi-factorial condition with risk factors such as maternal obesity, diabetes and hypertension [47,48] which are notably prevalent in our pooled cohort (See Table 2 for prevalence data).
Our analysis highlighted that NAFLD pregnancies herald multiple potentially undesirable maternal and fetal outcomes. Our study is the first meta-analysis on the topic of NAFLD in pregnancy and its outcomes and showing that NAFLD has a significant association with a past or current history of GDM, GHTN, pre-eclampsia and a range of other baseline characteristics associated with the metabolic syndrome. NAFLD in pregnancy is associated with fetal outcomes, with significantly higher odds of premature birth, a novel significant association with a history of abortion or miscarriage and large for gestational age birth. Moreover, the significantly elevated odds for current GHTN in NAFLD would promote further research to a past history of GHTN and pre-pregnancy hypertension which are likely associated. Data on these two areas is currently limited. The primary outcome was the demonstration of a myriad of diabetic, hypertensive associations and fetal complications. Multiple co-variates showed significant associations such as certain ethnic backgrounds and the interesting link with parity are all specific risk factors for NAFLD in the pregnancy state; this is a major strength of the analysis. The higher occurrence of preterm birth in NAFLD patients is possibly translated through the higher occurrence of DM and HTN in its various severities, which are known risk factors for prematurity [49]. The higher occurrence of past miscarriage or abortion is also related to obesity which is a stronger risk factor for miscarriage than DM [50].
A limitation of this analysis included studies that did not explicitly excluded concomitant hepatitis B/C (4 of 14), also none of the studies commented on the presence or absence of liver transplantation patients. Only one study commented on the presence of NASH subset of patients [19]. Various studies did not disclose meeting the clinical diagnosis criteria for NAFLD of demonstrating hepatic steatosis (imaging or biopsy), excluding significant alcohol and other causes of hepatic steatosis and the absence of co-existing chronic liver disease (Table 3) [51]. We were unable to assess publication bias as there was not a reasonable number of studies to undertake the analysis (a minimum of 10 studies are recommended for Egger’s regression analysis) [35]. Therefore, we are unsure of the impact of publication bias in this meta-analysis and given that there was substantial heterogeneity in some of the analyses the number of studies required to undertake an Egger’s regression and publication bias assessment would be substantially more than ten studies [35]. The strengths of this meta-analysis included a comprehensive literature search strategy which consisted of several electronic databases to ensure a broad range of literature from different regions of the world were included. A noteworthy strength was the low level of statistical heterogeneity found in many variables in the analysis. This highlights the consistency of the individual study results for each of the various outcomes assessed in this study. Furthermore, despite the observational nature of the studies included, the combination of the sample size and the quality of the studies allowed for moderate GRADE scores for clinical outcomes highlighting that the estimated effect is likely close to the true effect. Finally, we cannot be assured that in the individual studies all negative outcomes were reported accurately.
Current The Asian Pacific Association for the Study of the Liver, European Association for the Study of the Liver, and The American Association for the Study of Liver Diseases management guidelines for NAFLD do not discuss the disease in the pregnancy state. In high metabolic risk female patients, the pre-conception physician review is suggested to test prospective mothers with NAFLD or a past history of GDM or GHTN for the other associated comorbidities. This allows early management, setting weight targets and pharmacological therapy for GDM or GHTN. Further studies are needed to hypothesize the BMI target to recommend screening for NAFLD and GDM in pregnancy. However, a pregnant obese woman with GDM in the current or previous pregnancies would benefit from screening for NAFLD, and cardiovascular risk factors such as lipids profile and for hypertension. Conversely, a pregnant woman with the risk factors of obesity (BMI >30), current GHTN, AITD or Hispanic race should consider screening for NAFLD and GDM. Whilst screening for GDM is universally performed during the second trimester, screening for NAFLD post-partum is reasonable as it would allow patient education, lifestyle modification and importantly the potential to diagnose the cirrhotic subset of NASH patients to prevent further liver specific morbidity too. Given the sheer volume of NAFLD worldwide, further studies are needed to assess the ideal target in screening for each risk factor, to balance between clinical gain and financial and logistical limitations.
NAFLD in pregnancy is associated with substantial adverse maternal and fetal outcomes. Those with the condition should be educated immediately after diagnosis and be closely monitored before, during and after pregnancy. Long-term follow up studies are essential to help establish the potential association of current NAFLD with future development of pregnancy-related metabolic conditions. Future studies are also required to assess pregnant patients with NAFLD to guide the multidisciplinary management of these patients.
Notes
Authors’ contributions
Study concept and design: Hydar El Jamaly, Martin Weltman. Acquisition of data: Hydar El Jamaly, Guy D. Eslick. Analysis and interpretation of data: Hydar El Jamaly, Guy D. Eslick. Drafting of the manuscript: Hydar El Jamaly, Guy D. Eslick, Martin Weltman. Critical revision of the manuscript for important intellectual content: Hydar El Jamaly, Guy D. Eslick, Martin Weltman. Statistical analysis: Guy D. Eslick, Hydar El Jamaly. Study supervision: Martin Weltman, Guy D. Eslick.
Conflicts of Interest
The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
GRADE assessment of various outcomes and co-variates
Individual study characteristic
Abbreviations
AITD
autoimmune thyroid disease
BMI
body mass index
CI
confidence interval
DM
diabetes mellitus
GDM
gestational diabetes mellitus
GHTN
gestational hypertension
HELLP
haemolysis
LGA
large for gestational age birth
LSCS
low section caesarean section
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
OR
odds ratio
References
Article information Continued
Notes
Study Highlights
NAFLD during pregnancy is associated with multiple maternal diabetic and hypertensive complications. It is also associated with fetal complications such as premature birth and a large for gestational age birth. This information is essential to improve our understanding of NAFLD and pregnancy and guide future clinical care.