Non-alcoholic fatty liver disease increases risk of carotid atherosclerosis and ischemic stroke: An updated meta-analysis with 135,602 individuals
Article information
Abstract
Background/Aims
Non-alcoholic fatty liver disease (NAFLD) is associated with the development of cardiovascular disease. While existing studies have examined cardiac remodeling in NAFLD, there has been less emphasis on the development of carotid atherosclerosis and stroke. We sought to conduct a meta-analysis to quantify the prevalence, risk factors, and degree of risk increment of carotid atherosclerosis and stroke in NAFLD.
Methods
Embase and Medline were searched for articles relating to NAFLD, carotid atherosclerosis, and stroke. Proportional data was analysed using a generalized linear mixed model. Pairwise meta-analysis was conducted to obtain odds ratio or weighted mean difference for comparison between patients with and without NAFLD.
Results
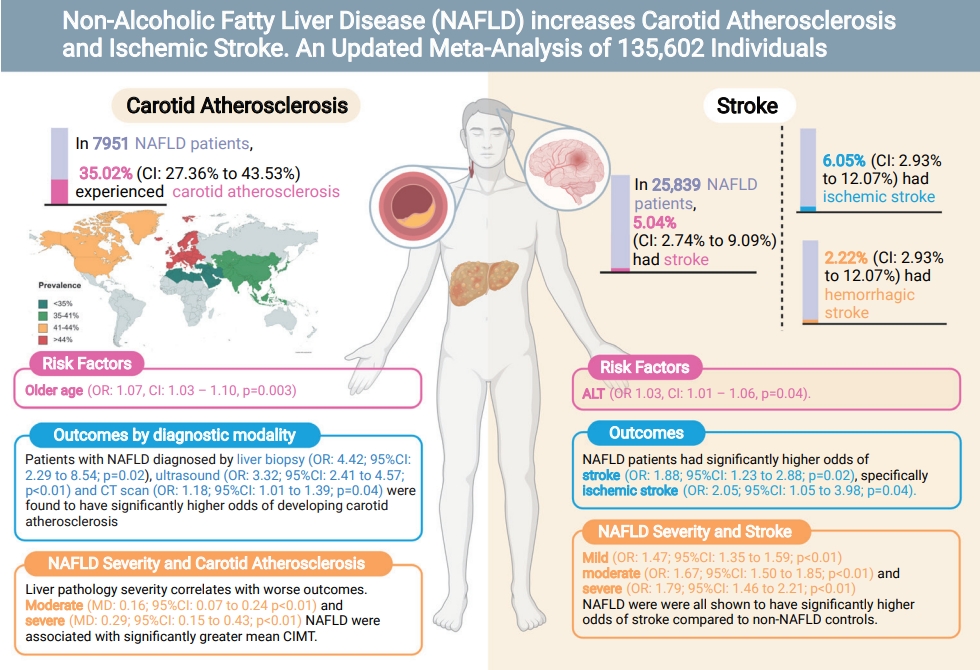
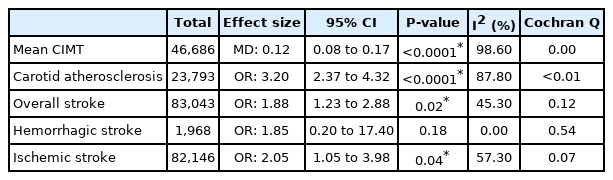
From pooled analysis of 30 studies involving 7,951 patients with NAFLD, 35.02% (95% confidence interval [CI], 27.36–43.53%) had carotid atherosclerosis with an odds ratio of 3.20 (95% CI, 2.37–4.32; P<0.0001). Pooled analysis of 25,839 patients with NAFLD found the prevalence of stroke to be 5.04% (95% CI, 2.74–9.09%) with an odds ratio of 1.88 (95% CI, 1.23–2.88; P=0.02) compared to non-NAFLD. The degree of steatosis assessed by ultrasonography in NAFLD was closely associated with risk of carotid atherosclerosis and stroke. Older age significantly increased the risk of developing carotid atherosclerosis, but not stroke in NAFLD.
Conclusions
This meta-analysis shows that a stepwise increment of steatosis of NAFLD can significantly increase the risk of carotid atherosclerosis and stroke development in NAFLD. Patients more than a third sufferred from carotid atherosclerosis and routine assessment of carotid atherosclerosis is quintessential in NAFLD.
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease affecting 22% to 30% of the global population [1,2]. NAFLD is characterized by the evidence of hepatic steatosis, either by imaging or histology, and the absence of secondary causes of hepatic lipid accumulation such as excessive alcohol consumption, long-term use of steatogenic medications, or monogenic hereditary disorders [3]. NAFLD encompasses a wide spectrum of disease ranging from simple steatosis to nonalcoholic steatohepatitis, a more progressive and advanced form of the disease characterized by inflammation, ballooning, and hepatocellular injury [4], which may subsequently progress to liver cirrhosis [5] and liver cancer [6]. The presence of NAFLD can result in a host of complications including the development of cardiovascular disease, extrahepatic or hepatic malignancy [7], and depression [8].
In NAFLD, the presence of chronic energy surplus causes lipo-toxicity, cell death, and inflammation [9], which can lead to the development of atherosclerosis [10] in the coronary, carotid, and peripheral arteries [11]. While current studies have predominantly focused on the development and progression of cardiac-related disease in NAFLD, the potential implications of carotid atherosclerosis are severe and predispose to the development of stroke. Stroke is the third-leading cause of disability and the second-leading cause of death worldwide [12]. Although common modifiable risk factors of stroke such as smoking, diet, and physical inactivity have been studied, there is still a limited understanding of stroke beyond its traditional risk factors [13], especially in the presence of metabolic dysfunction. Recent studies have found NAFLD to be an independent risk factor of stroke [14].
Current meta-analyses have focused on the association of NAFLD with coronary atherosclerosis and cardiovascular diseases [15-18]. However, a systematic analysis of the prevalence, risk factors, degree of steatosis in NAFLD and carotid atherosclerosis or stroke remains limited. Hence, this paper seeks to conduct an updated analysis of the associations of NAFLD with mean carotid intima-media thickness (CIMT), carotid atherosclerosis, stroke risk, and factors associated with these developments, with further subgroup analyses based on NAFLD severity and diagnostic modalities.
MATERIALS AND METHODS
Search strategy and inclusion criteria
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for its synthesis. Two electronic databases, MEDLINE, and Embase, were used to search all available records till 21st October 2021. The search strategy used search terms including ‘nonalcoholic fatty liver disease’, ‘hemorrhagic stroke’, ‘ischemic stroke’, ‘CIMT’, and other related terms in titles and abstracts. The full search strategy is included in Supplementary Material 1. References were imported into Endnote X9 for duplicate removal. References of the included articles and previous meta-analysis were also manually screened to ensure a comprehensive search.
Eligibility and selection criteria
Four authors (KEC, JQ, ASPT, and JX) independently screened abstracts and conducted full-text reviews to check the eligibility for inclusion, with disputes being resolved by obtaining the consensus of a fifth independent author (CHN). Only original articles were included, and reviews, commentaries, and editorials were excluded. Studies that were written or translated into the English language were included. NAFLD was defined as evidence of hepatic steatosis, either by imaging or histology and lack of secondary causes of hepatic fat accumulation such as significant alcohol consumption, long-term use of steatogenic medication or monogenic hereditary disorders [3]. Diagnosis of NAFLD was determined either from invasive methods, such as liver biopsy, or non-invasive methods including ultrasound and computed tomography (CT) scan. Blood-based diagnosis including NAFLD fibrosis score (NFS) was excluded from the analysis. Studies were included if they fulfilled the following criteria: one of the following outcomes 1) mean CIMT; 2) increased in CIMT, stenosis or plaques; and 3) stroke. Mean CIMT was defined as the average width of the intima-media layer of the carotid artery assessed by Duplex ultrasonography (DUS) [19]. Carotid arteriosclerosis was defined as the presence of carotid plaques/stenosis or an increase in CIMT. Stroke was defined as the presence of either ischemic and hemorrhagic stroke. For multiple studies inferring results from the same databases, duplicates were removed and only the most updated studies were included for analysis.
Data extraction and outcomes
NAFLD severity was graded according to ultrasound findings to maintain homogeneity. Mild NAFLD was defined as a slight diffuse increase in the hepatic parenchyma echogenicity with normal visualisation of the diaphragm and portal veins. Moderate NAFLD was defined as moderate diffuse increase in hepatic echogenicity, with slight impaired visualisation of the diaphragm and portal veins. Severe NAFLD was defined as a marked increase in hepatic echogenicity with poor or no visualisation of the diaphragm and portal veins [20,21]. Two pair of authors (KEC and ASPT; JQ and JL) independently extracted data including but not limited to 1) study characteristics such as: author, year, country, study design; 2) patient characteristics such as sample size, age, male gender, diagnostic criteria, severity of NAFLD, body mass index, presence of metabolic conditions including dyslipidemia and hypertension; and 3) clinical outcomes. Transformation of values was carried out using pre-existing formulae, in which mean and standard deviations were estimated from median and range using the widely adopted formula by Wan et al. [22] Blinded checking of the data by the authors was conducted to ensure accuracy of the data extracted and discrepancies in data were resolved through consensus.
Statistical analysis
All analyses were conducted in R studio (version 5.0.0; The R Foundation, Indianapolis, IN, USA). A P-value ≤0.05 was considered as the threshold for statistical significance. Statistical heterogeneity was assessed via I2 and Cochran’s Q test values, where I2 values of 25%, 50% and 75% represented low, moderate and high degrees of heterogeneity, respectively [23,24]. A random effect model was used in all analyses regardless of heterogeneity measures as evidence has demonstrated more robust effect estimates with random effect compared to fixed-effect models [25,26]. An analysis of proportions was pooled using a generalized linear mixed model with Clopper-Pearson intervals [27,28]. To assess risk factors, a generalized mix model with a logit link and inverse variance weightage were used to derived the odds ratio (OR) [29]. Bivariate analysis of dichotomous variables was analysed in Paul Mantel ORs with and mean difference (MD) with inverse variance for continuous variables. A subgroup analysis was performed based on NAFLD diagnostic modality (ultrasound, CT, biopsy) and geographical location in the analysis of CIMT. Where possible, a sensitivity analysis was also performed on severity of NAFLD (i.e., mild, moderate, severe) based on ultrasound evaluation. Publication bias was examined based on asymmetry of the funnel plots (Supplementary Fig. 1) where sufficient studies were present (n>10) with Begg regression.
Quality assessment
Quality assessment of included articles was done with the Joanna Briggs Institute (JBI) critical appraisal tool (JBI Collaboration, Adelaide, Australia) [30]. The JBI assessment rates the quality of cohort studies on the premises of appropriateness of sample frame, sampling method, adequacy of sample size, data analysis, methods for identification and measurement of relevant condition, statistical analysis, and response rate adequacy.
RESULTS
Summary of included articles
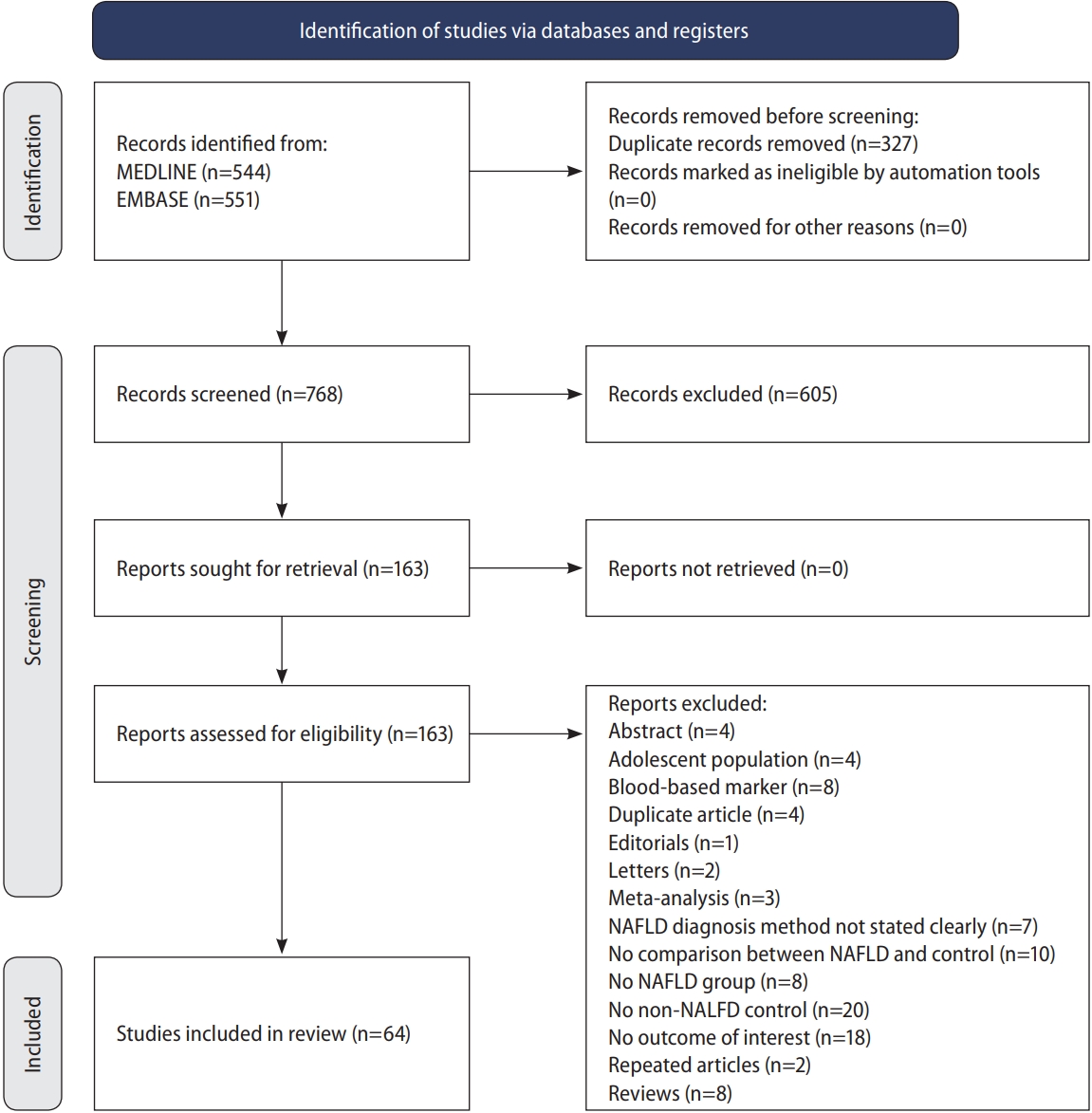
The initial search from MEDLINE and Embase yielded a total of 1,095 articles. After the removal of 327 articles duplicated during the title abstract sieve, 768 articles were remained for abstract screening and a final total of 64 studies conducted between 1998 to 2019 were included in the meta-analysis (Fig. 1). The studies included were conducted in various countries including Algeria [31], China [32-39], Croatia [40], Egypt [41,42], Greece [43,44], India [45-49], Iran [50-57], Italy [58-66], Japan [67,68], Malaysia [69,70], Romania [71], Serbia [72], South Korea [73-78], Spain [79,80], Taiwan [81], Turkey [82-92], USA [93,94]. Additionally, one study [95] was a multicentre study based in Egypt [43]. A total of 135,602 patients were included in our analysis with 47,322 patients with NAFLD and 88,280 non-NAFLD controls. NAFLD defined by liver biopsy [44,82,84,86,87,90,92] was found in seven studies, and by non-invasive methods, including ultrasonography [31-43,45-81,83,85,86,88,89,91,93] in 57 studies and CT scan [94] in one study. All studies were assessed to have a high (n=46) or moderate (n=18) quality based on the JBI Critical Appraisal Checklist assessment tool (Supplementary Table 1). There was no evidence of publication bias (Fig. 2) with Begg regression (P=0.787).
Carotid atherosclerosis
Prevalence and risk factors of carotid atherosclerosis
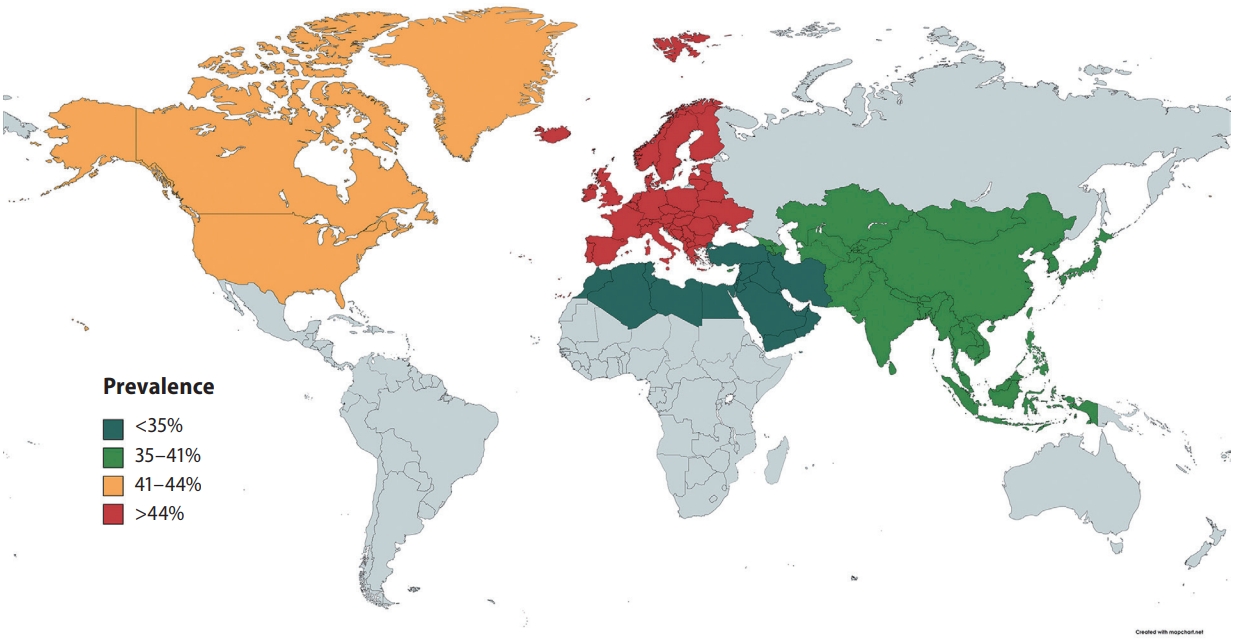
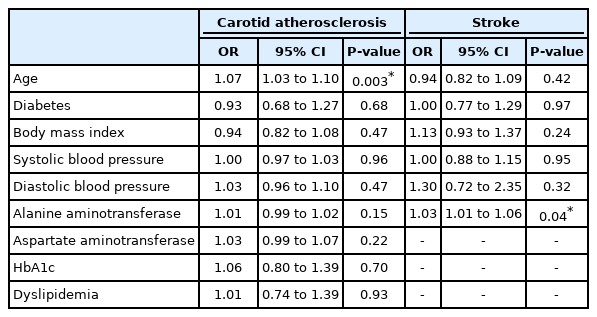
In pooled analysis of 7,951 patients with NAFLD, the prevalence of carotid atherosclerosis in NAFLD was found to be 35.02% (95% confidence interval [CI], 27.36–43.53%). When a subgroup analysis by geographical regions was conducted, the prevalence of carotid atherosclerosis in NAFLD was found to be the highest in Europe (44.72%; 95% CI, 31.02–59.28%; Fig. 3), followed by North America (41.02%; 95% CI, 37.50–44.63%), Asia (35.89%; 95% CI, 24.61–48.98%), and the lowest in the Middle East (19.21%; 95% CI, 12.58–28.21%). Table 1 summarizes the risk factor of carotid atherosclerosis in NAFLD. The presence of older age (OR, 1.07; 95% CI, 1.03–1.10; P=0.003) increases the risk of carotid atherosclerosis in NAFLD.
Comparative outcomes
A summary of comparative results can be found in Table 2. When compared to non-NAFLD controls, patients with NAFLD were shown to have significantly higher risk of carotid atherosclerosis (OR, 3.20; 95% CI, 2.37–4.32; P<0.0001). Subgroup analysis by diagnostic modality similarly demonstrated that patients with NAFLD diagnosed by liver biopsy (OR, 4.42; 95% CI, 2.29–8.54; P=0.02), ultrasound (OR, 3.32; 95% CI, 2.41–4.57; P<0.01), and CT scan (OR, 1.18; 95% CI, 1.01–1.39; P=0.04) had significantly higher risk of developing carotid atherosclerosis as compared to non-NAFLD controls. Patients with NAFLD were also found to have a significantly greater mean CIMT than those without NAFLD (MD, 0.12; 95% CI, 0.08–0.17; P<0.0001). Subgroup analysis of mean CIMT based on the severity of NAFLD revealed that liver pathology severity correlates with the worse outcomes. Patients suffering from moderate NAFLD (MD, 0.16; 95% CI, 0.07–0.24; P<0.01) and severe NAFLD (MD, 0.29; 95% CI, 0.15–0.43; P<0.01) had significant increase in mean CIMT but not in mild NAFLD (MD, -0.04; 95% CI, -0.28 to 0.20; P=0.76).
Stroke
Prevalence and risk factors of stroke
In pooled analysis of 25,839 individuals with NAFLD, the incidence of stroke in NAFLD was found to be 5.04% (95% CI, 2.74–9.09%). Specifically, the incidence of ischemic stroke in NAFLD was 6.05% (95% CI, 2.93–12.07%) while the incidence of hemorrhagic stroke was found to be 2.22% (95% CI, 0.22–18.77%). In NAFLD, there was no significant factor affecting the presence of stroke aside from an increase level of alanine aminotransferase (ALT) (OR, 1.03; 95% CI, 1.01–1.06; P=0.04).
Comparative outcomes
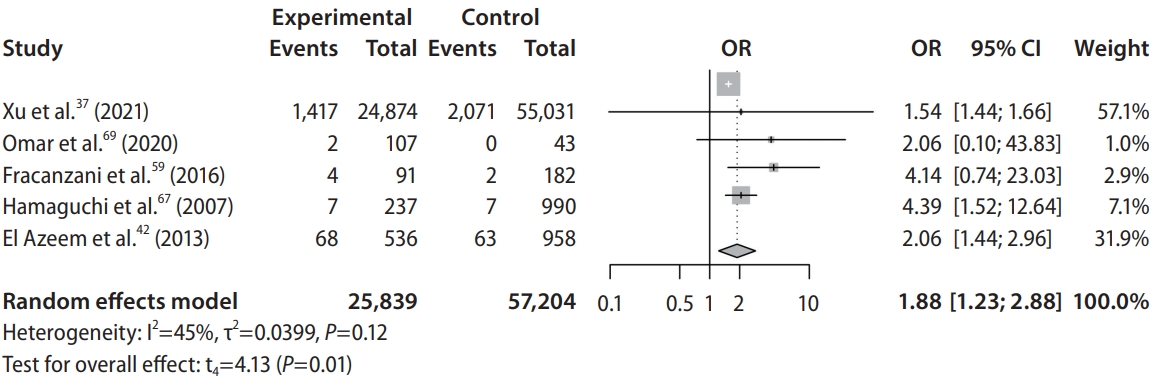
Patients with NAFLD were shown to have significantly higher risk of developing stroke (OR, 1.88; 95% CI, 1.23–2.88; P=0.02; Fig. 2, Table 2) when compared to non-NAFLD controls. Specifically, patients with NAFLD were found to have significantly higher risk of developing ischemic stroke (OR, 2.05; 95% CI, 1.05–3.98; P=0.04) when compared to non-NAFLD controls. However, comparisons between NAFLD and non-NAFLD controls found that there was no significant difference in the risk of developing hemorrhagic stroke (OR, 1.85; 95% CI, 0.20–17.40; P=0.18). Subgroup analysis classified by the severity of NAFLD found that patients suffering from mild (OR, 1.47; 95% CI, 1.35–1.59; P<0.01), moderate (OR, 1.67; 95% CI, 1.50–1.85; P<0.01), and severe (OR, 1.79; 95% CI, 1.46–2.21; P<0.01). NAFLD were all shown to have significantly higher risk of developing stroke when compared to non-NAFLD controls.
DISCUSSION
The presence of NAFLD has been associated with an increase in atherogenic dyslipidemia [96,97] leading to the development of cardiovascular complications such as myocardial infarctions [98] and cerebrovascular accident [7]. While previous studies have demonstrated the association between NAFLD, carotid atherosclerosis [81] and increased stroke risks [37], the current findings further expand on the published literature by providing a contemporaneous analysis on the prevalence of carotid atherosclerosis and stroke in NAFLD, risks factors, and the influence of NAFLD severity and diagnostic modality. Significantly, the degree of steatosis in NAFLD can influence the progression of CIMT and stroke, with severe steatosis resulting in the highest odds of events.
In our meta-analysis, the prevalence of carotid atherosclerosis in NAFLD was found to be 35.02% (95% CI, 27.36– 43.53%) and stroke was 5.04% (95% CI, 2.74–9.09%). Subgroup analysis based on geographical regions found the prevalence of carotid atherosclerosis in NAFLD to be highest in the West. While the geographical regions are not a representation of ethnicity, the prevalence of CIMT have been found to be higher in the Caucasian population [99]. In the analysis of risk factors, the presence of hyperlipidemia and diabetes did not significantly increase the risk of stroke or carotid atherosclerosis in NAFLD. Only older age increased the risk of carotid atherosclerosis while ALT increased the risk of stroke in NAFLD. These results, however, should be interpreted with caution as these findings do not discount the possibility that hyperlipidemia and diabetes may have not been significant due to insufficient statistical power arising from the limited sample size in the risk factor analysis. However, the presence of NAFLD could potentially increase the risk of stroke and CIMT independently with more severe steatosis significantly increasing the rate of events.
While the presence of carotid atherosclerosis does not necessarily translate into an event of ischemic stroke, the presence of carotid atherosclerosis increases the risk of ischemic stroke by 20% [100]. The presence of carotid plaques has also been associated with cerebral atrophy and reduced cognitive ability [101]. In turn, while more than a third of patients with NAFLD sufferred from carotid atherosclerosis, only 5.04% (95% CI, 2.74–9.09%) of patients with NAFLD suffered from a stroke with twice the risk (OR, 1.88; 95% CI, 1.23–2.88; P=0.02) compared to those without NAFLD. Specifically, the presence of NAFLD was found to result in significantly higher risk of developing ischemic stroke (OR, 2.05; 95% CI, 1.05– 3.98; P=0.04) but not in hemorrhagic stroke (OR, 1.85; 95% CI, 0.20–17.40; P=0.18). While the incidence of stroke in NAFLD is rather minute, the presence of NAFLD resulted in twice the risk of stroke and the implications of which can be devastating [102]. Additionally, it is important that we do not undermine the presence of subclinical carotid atherosclerosis, evidently more prevalent in NAFLD as it represents prime therapeutic targets to prevent morbidity and mortality associated with clinically evident disease (i.e., stroke).
Prevailing guidelines by the American Association for the Study of Liver Diseases (AASLD) [103] and the European Association for the Study of the Liver (EASL) [104] have highlighted the importance of cardiovascular risk evaluation in NAFLD. However, the use of CIMT for NAFLD has yet to be endorsed or routinely recommended for NAFLD. Yet, the DUS is a safe, easy, and cost-effective screening investigative tool that can be routinely employed in the clinic [105,106], and has a sensitivity and specificity of 90% and 94% for carotid artherosclerosis [107]. Additionally, the DUS can double as a screening modality for coronary artery disease (CAD) with a respective sensitivity and specificity of 78% and 75% for CAD [108]. CAD is a known complication of NAFLD, and recent estimates suggest the prevalence of CAD to be 38.7% and 55.4%, respectively [10]. With the strong correlation between coronary and carotid atherosclerosis with NAFLD, future studies should assess the cost effectiveness and viability for routine DUS evaluation for NAFLD.
Strengths and limitations
This study provides an up-to-date comprehensive assessment of the association between NAFLD, carotid atherosclerosis and increased stroke risk, including the prevalence, risk factors, and severity of steatosis. However, there are several limitations. Although liver biopsy has higher sensitivity and specificity in the diagnosis of NAFLD, majority of studies utilized non-invasive, imaging-based investigations rather than biopsy to diagnose NAFLD. In addition, while modalities such as CT or Fibroscan may be more accurate in grading NAFLD severity, grading of NAFLD severity was limited to ultrasonographic findings to maintain homogeneity with majority of studies utilizing ultrasonography (89.1%) and is similar to our previous meta-analysis [109]. We were also unable to assess the effects of stage of fibrosis on CIMT or stroke due to lack of granularity in the reported data. Lastly, the included studies were largely retrospective and are subjected to inherent limitations of the study design such as selection bias.
Conclusions
Patients with NAFLD were found to be associated with increased carotid atherosclerosis and stroke prevalence. While the prevalence of stroke in NAFLD may be low, the severe consequences highlight the importance for CIMT evaluation in NAFLD. Routine screening for carotid atherosclerosis, guided by the severity of hepatic steatosis, among patients with NAFLD might aid in the reduction of stroke.
Notes
Authors’ contributions
Conceptualization: Mark Muthiah, Arun J Sanyal, Cheng Han Ng
Data curation: Ansel Shao Pin Tang, Kai En Chan, Jingxuan Quek, Cheng Han Ng
Formal analysis: Ansel Shao Pin Tang, Kai En Chan, Jingxuan Quek, Jieling Xiao, Phoebe Tay
Supervision: Margaret Teng, Keng Siang Lee, May Zin Myint, Benjamin Tan, Vijay K Sharma, Darren Jun Hao Tan, Wen Hui Lim, Daniel Huang, Nicholas WS Chew, Mohammad Shadab Siddiqui, Mark Muthiah, Arun J Sanyal, Cheng Han Ng
Validation: Ansel Shao Pin Tang, Kai En Chan, Jingxuan Quek, Jieling Xiao, Phoebe Tay, Margaret Teng, Keng Siang Lee, May Zin Myint, Benjamin Tan, Vijay K Sharma, Darren Jun Hao Tan, Wen Hui Lim, Daniel Huang, Apichat Kaewdech, Nicholas WS Chew, Mohammad Shadab Siddiqui, Mark Muthiah, Arun J Sanyal, Cheng Han Ng
Writing, original draft: Ansel Shao Pin Tang, Kai En Chan, Jingxuan Quek, Cheng Han Ng
Writing, review, and editing: Ansel Shao Pin Tang, Kai En Chan, Jingxuan Quek, Jieling Xiao, Phoebe Tay, Margaret Teng, Keng Siang Lee, Snow Yunni Lin, May Zin Myint, Benjamin Tan, Vijay K Sharma, Darren Jun Hao Tan, Wen Hui Lim, Daniel Huang, Apichat Kaewdech, Nicholas WS Chew, Mohammad Shadab Siddiqui, Mark Muthiah, Arun J Sanyal, Cheng Han Ng
All authors have read and approved the final version of the manuscript for submission.
Conflicts of Interest
AJS is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect and Galmed. He has served as a consultant to Astra Zeneca, Nitto Denko, Enyo, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Norvatis. He receives royalties from Elsevier and UptoDate. MN has been on the advisory board for 89BIO, Gilead, Intercept, Pfizer, Novo Nordisk, Blade, EchoSens, Fractyl, Terns, Siemens and Roche diagnostic; MN has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Novartis, Pfizer, Shire, Viking and Zydus; MN is a minor shareholder or has stocks in Anaetos, Rivus Pharma and Viking.
All other authors do not have any conflict of interest to declare.
Acknowledgements
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures and tables has not been previously published and that the manuscript is not under consideration elsewhere. This research meets the ethical guidelines, including adherence to the legal requirements of the study country.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Search strategy
Summary of included articles
Funnel plots of publication bias.
Abbreviations
AASLD
the American Association for the Study of Liver Diseases
ALT
alanine aminotransferase
CAD
coronary artery disease
CI
confidence interval
CIMT
carotid intima-media thickness
CT
computed tomography
DUS
Duplex ultrasonography
EASL
the European Association for the Study of the Liver
JBI
Joanna Briggs Institute
MD
mean difference
NAFLD
non-alcoholic fatty liver disease
NFS
NAFLD fibrosis score
OR
odds ratio
References
Article information Continued
Notes
Study Highlights
• Out of 7,951 NAFLD patients, 35.02% had carotid atherosclerosis with an OR of 3.20.
• The prevalence of stroke in NAFLD patients was 5.04% with an OR of 1.88.
• The routine assessment of carotid atherosclerosis is quintessential in NAFLD.