Nonalcoholic fatty liver disease and non-liver comorbidities
Article information
Abstract
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease characterized by excess fat accumulation in the liver. It is closely associated with metabolic syndrome, and patients with NAFLD often have comorbidities such as obesity, type 2 diabetes mellitus, and dyslipidemia. In addition to liver-related complications, NAFLD has been associated with a range of non-liver comorbidities, including cardiovascular disease, chronic kidney disease, and sleep apnea. Cardiovascular disease is the most common cause of mortality in patients with NAFLD, and patients with NAFLD have a higher risk of developing cardiovascular disease than the general population. Chronic kidney disease is also more common in patients with NAFLD, and the severity of NAFLD is associated with a higher risk of developing chronic kidney disease. Sleep apnea, a disorder characterized by breathing interruptions during sleep, is also more common in patients with NAFLD and is associated with the severity of NAFLD. The presence of non-liver comorbidities in patients with NAFLD has important implications for the management of this disease. Treatment of comorbidities such as obesity, type 2 diabetes mellitus, and dyslipidemia may improve liver-related outcomes in patients with NAFLD. Moreover, treatment of non-liver comorbidities may also improve overall health outcomes in patients with NAFLD. Therefore, clinicians should be aware of the potential for non-liver comorbidities in patients with NAFLD and should consider the management of these comorbidities as part of the overall management of this disease.
INTRODUCTION
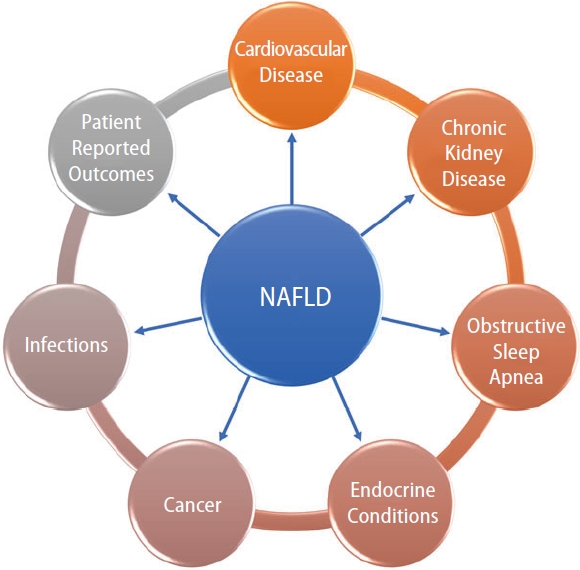
As the incidence and prevalence of nonalcoholic fatty liver disease (NAFLD) continues to increase worldwide, the association of NAFLD with other comorbid conditions is an area of increasing interest and research [1-9]. In several studies, NAFLD has been found to be an independent risk factor for adverse outcomes, including mortality [10], even after controlling for other known risk factors. However, the relationship of NAFLD to other comorbid conditions is still under investigation, especially when trying to understand whether these conditions coexist or if one causes the other. Furthermore, the presence of fibrosis complicates this relationship as when fibrosis is present, it becomes the number one predictor of mortality [11-19]. Nonetheless, having an understanding of what comorbidities are often associated with NAFLD is important so that proper treatment can be forthcoming. Therefore, the following will provide a brief review of these conditions (Fig. 1) and the current evidence regarding each association.
CARDIOVASCULAR DISEASE
Ischemic heart disease
The most common cause of death in patients with NAFLD is the spectrum of cardiovascular disease (CVD) comprising coronary artery disease, angina, and ischemic stroke. The incidence of CVD in NAFLD has been estimated to be as high as 100.6 per 1,000 person-years [20]. Though it appears clear that the two conditions are associated, the proof for NAFLD being an independent cause of CVD has not been borne out by the evidence [21]. The absence of a causative link, however, may be due to a lack of data in stratifying CVD in relation to the level of fibrosis. NAFLD does appear to increase the overall risk of CVD, but it is not yet clear if it increases mortality caused by CVD. A meta-analysis of 16 studies showed that NAFLD significantly increased the risk of non-fatal cardiovascular events with an odds ratio (OR) of 2.52 when compared to patients without NAFLD, but no significant relationship was found between NAFLD and the risk of fatal cardiovascular outcomes. However, if severe NAFLD was assessed, as defined by fatty liver on imaging with either increased gamma-glutamyltransferase (GGT) or elevated NAFLD fibrosis score or positron emission tomography showing increased fluorodeoxyglucose (FDG) uptake or worsening fibrosis on pathology, then there was a higher risk of CVD mortality with an OR of 3.28 when compared to patients without NAFLD [22].
Pathophysiologically, the metabolic syndrome inflicts widespread end-organ damage which manifests as CVD and NAFLD. The mechanism is thought to be related to the accumulation of visceral and ectopic fat leading to the production and release of fat-derived toxic metabolites. These metabolites trigger systemic and local inflammation ultimately resulting in the progression of both NAFLD and CVD [23].
As the mechanisms are similar, the treatment guidelines are shared among the diseases. The American Heart Association has released the “Life’s Simple 7” guidelines with a stated goal of reducing deaths from CVD and stroke by 20%. A recent study conducted among patients with NAFLD using Life’s Simple 7 guidelines did find that if all NAFLD subjects achieved an ideal rating on all 7 of the health metrics, 66% of all-cause deaths and 83% of cardiovascular (CV) deaths were preventable. In fact, among NAFLD subjects, lack of glycemic control (adjusted population attributable fraction [PAF] =28.3% all-cause; 38.1% CV) and hypertension (adjusted PAF of 23% all-cause; 52.8% CV) were the largest mortality contributors while obtaining ideal physical activity level provided an adjusted PAF=13.9% all-cause and 13.8% CV mortality [24].
A Mediterranean style diet has also been proposed as an intervention that may help decrease the incidence of both NAFLD and CVD [25].
However, just recently, the American Health Association updated their Life Simple 7 guidelines to include sleep as a new metric (Life’s Simple 8 guidelines) [26] as well as updating their diet recommendations to include more food groups such as what is found in a Mediterranean style diet and to use non-high density lipoprotein (HDL) cholesterol measurement for lipid quantification. In this light, a recently published study looked at both sleep and fatigue and their impact on NAFLD mortality. Investigators reported that adults with NAFLD and fatigue experienced 2.3-fold higher mortality than adults with NAFLD but without fatigue. In addition, depression, sleep disturbance and CVD were all major predictors of fatigue, while not having a sleep disturbance had an inverse relationship with mortality [27]. As such, the association between NAFLD and CVD is complex which requires a systematic treatment approach as outlined in several recent guidelines [28-35].
Congestive heart failure
As the end-stage phenotype of multiple cardiac conditions, congestive heart failure (CHF) is a widespread threat. Associations have been drawn between the presence of NAFLD and CHF. The risk of incident heart failure in patients with NAFLD is higher than patients without NAFLD with an estimated hazard ratio of 1.75, according to a study from Sweden looking at 10,422 patients over a median follow-up period of 13.6 years [36]. Increased epicardial fat in patients diagnosed with fatty liver leads to abnormal energy metabolism, especially in the left ventricle, despite seemingly normal systolic and diastolic function as measured by echocardiography [37]. Positive correlations are seen between hepatic and myocardial triglyceride content as measured by magnetic resonance. Rijzewijk et al. [38] showed that greater amounts of myocardial fat deposition contribute to left ventricular (LV) diastolic dysfunction, predisposing to heart failure with preserved ejection fraction [38].
In the other well-known phenotype of CHF, heart failure with reduced ejection fraction, NAFLD appears to be an independent risk factor. Even after accounting for obesity, insulin resistance and a suboptimal diet, the presence of NAFLD remains an independent factor contributing to a lower ejection fraction [39]. Using ultrasound and echocardiography, Trovato et al. [39] performed multiple linear regression to compare the presence of fatty liver with ejection fraction and found a statistically significant negative correlation. This deeply concerning finding brings into perspective the much greater risk that these patients with NAFLD face. Further complicating the picture is the population that is not obese yet has underlying fatty liver. High clinical suspicion would be required at the frontlines to find these “lean NAFLD” patients and ensure adequate cardiovascular risk stratification in this population.
The prevalence of NAFLD is 36% in patients with, heart failure with reduced ejection fraction (HFrEF), significantly higher than in the general population. The combination of NAFLD and HFrEF may be particularly troublesome, as these patients are on average younger and have a higher body-mass index, larger LV mass, and greater fibrosis in the LV myocardium [40]. The changes are not all morphologic, as these patients with NAFLD in addition to HFrEF have higher rates of in-hospital and post-discharge all-cause mortality. Advanced fibrosis due to NAFLD is a specific cause of even greater all-cause mortality [41].
Valvular heart disease
Studies have examined the presence of increased cardiac valvular calcification with NAFLD prevalence. Coexisting sclerosis of the aortic valve (AVS) along with calcification of the mitral annulus (MAC) appears to have the strongest correlation, while patients without any valvular calcification are the least likely to have NAFLD. Isolated AVS and isolated MAC have an intermediate probability [42]. These associations are present independent of diabetes, kidney disease, medications and even echocardiographic values. Along with valvular calcification, suboptimal glycemic control and advancing kidney disease were the other independent predictors of valvular calcification, implying common causative pathways [43].
Treatment of valvular heart disease may also become more complicated. Anticoagulants like warfarin are frequently indicated in these patients to prevent thromboembolic events. Patients with NAFLD along with valvular disease are seen to require higher doses of warfarin and even then, they are less likely to stay in the therapeutic range as compared to patients without NAFLD [44].
Ischemic stroke
NAFLD appears to increase the frequency of ischemic stroke though the evidence for it being a potential causative factor has been conflicting. Some earlier smaller studies had not shown a clear association between the two entities [45,46]. However, according to a study from Sweden by Simon et al. [36], patients with NAFLD have a significantly increased risk of incident stroke when compared to patients without NAFLD (hazard ratio of 1.58). A large meta-analysis published in 2022 looking at 64 studies from 1998 to 2016 showed that the more advanced the NAFLD, the higher the risk of ischemic stroke. Mild NAFLD had an OR of 1.47 when compared to ischemic stroke, while moderate NAFLD had an OR of 1.67 and severe NAFLD had an OR of 1.79. Mild, moderate, and severe NAFLD were assessed with the degree of hepatic echogenicity on ultrasound. The authors felt that the data was conclusive enough to suggest the use of carotid intima-media thickness (CIMT), assessed by duplex ultrasonography, as a screening tool for NAFLD [47]. While CIMT is not yet being used by clinicians to check for NAFLD, the relationship does bring into perspective the close ties shared by these conditions.
A smaller study from Korea suggests that the assessment of steatosis alone may not adequately predict risk. It concludes that fibrosis specifically, and not necessarily the degree of steatosis, is what increases the risk of ischemic stroke [48]. The association does not seem to differ based on ethnicity or the type of ischemic stroke [49], though hemorrhagic stroke does not seem to have any relationship with the presence of NAFLD [47].
It does appear that patients who present with an ischemic stroke are more likely to have underlying NAFLD. A recent study from Japan reports that the frequency of NAFLD is nearly 40% in patients with stroke but only 26.4% in the general Japanese population [50]. From a clinical standpoint, patients who have been diagnosed with a new ischemic stroke should have closer follow-up regarding the status of their liver function, as this follow-up is not routinely done at present.
Specific phenotypes of ischemic stroke caused by NAFLD have been considered. Large artery atherosclerosis and small vessel occlusions are most commonly seen in stroke patients with NAFLD, whereas a cardioembolic etiology is less commonly found [51]. Brainstem infarctions may also be more common in this patient population and have a higher risk of progression even after adjusting for comorbidities [52].
Atrial fibrillation
An arrhythmia with already high prevalence in the general population, a diagnosis of NAFLD appears to push it higher. A prospective study from Finland on the Observational Pharmaco-Epidemiology Research & Analysis (OPERA) cohort looked at nearly 1,000 patients and established an independent association between the two conditions even after adjusting for age, sex and the presence of diabetes. The increase in risk was found to be nearly two-fold [53]. Though not directly assessing NAFLD, the Framingham study of 3,700 patients found that higher liver enzymes (aspartate transaminase and alanine transaminase) did correlate with an increased risk of incident atrial fibrillation [54]. At least 4 studies from 2014 to 2017 did suggest that elevated GGT levels were also independently associated with the development of atrial fibrillation. A review on the topic looking at 14 studies and 3 meta-analyses found one study that did not show an association between NAFLD and atrial fibrillation, while all the others suggested that NAFLD is associated with an increased risk of developing atrial fibrillation [55].
Ventricular arrhythmias
Other more immediately dangerous arrhythmias are also being linked to the presence of NAFLD. An excessively prolonged corrected QT (QTc) interval on electrocardiography can often degenerate into ventricular tachyarrhythmias and has been associated with sudden cardiac death [56]. Interestingly, the degree of NAFLD has been found to increase the QTc interval on patient EKGs. A large study of over 30,000 patients from Taiwan found that mild NAFLD increased QTc intervals by 2.55 ms and severe NAFLD increased it by 12.13 ms [57]. Smaller studies have confirmed this association in other parts of the world [58,59]. Clearly, patients with NAFLD would benefit from having a lower threshold for undergoing rhythm monitoring if symptomatic though the evidence does not yet support screening for arrhythmias in NAFLD.
Impact of dyslipidemia treatment
Contrary to what one may hope, treatment of dyslipidemia has not been found to improve NAFLD, though newer targets in the pipeline may be able to alter disease progression. In a study of 2,566 patients, traditional antidyslipidemic treatment, including hydroxy-methyl-glutaryl-coenzyme A reductase (HMG-CoA) reductase inhibitors, did not improve mortality or major adverse cardiovascular events in NAFLD [60]. It is possible that traditional therapies do not account for the specific phenotype of dyslipidemia that exists in NAFLD. A higher plasma apolipoprotein B to apolipoprotein A1 ratio has been found in patients with NAFLD even after taking obesity into account. Patients with NAFLD also have smaller low-density lipoprotein (LDL) particle size.
It is thought that these patients may require different treatment targets, which are currently being researched. Specifically, increasing hepatic fat metabolism is the proposed mechanism of action of resmetirom (MGL-3196), a selective thyroid hormone receptor agonist. This oral medication has shown increased reduction of hepatic fat as measured by magnetic resonance imaging (MRI) in phase 2 clinical trials though a clear mortality benefit is not yet evident [61]. Phase 3 trials confirm that resmetirom is as safe and as well tolerated as placebo while significantly improving liver transaminases and fibrosis biomarkers in addition to proton-density fat fraction on MRI [62].
Though PCSK9 inhibitors such as alirocumab and evolocumab are coming into more widespread use, these medications have not yet been shown to improve NAFLD. However, studies have shown that specific gene variants of PCSK7 have been associated with higher levels of inflammation in the liver along with higher transaminases [63]. Specifically targeting PCSK7 may be able to target NAFLD and its downstream deleterious effects [64].
CHRONIC KIDNEY DISEASE
Much research has been done into the risk of incident chronic kidney disease and nonalcoholic fatty liver disease. A 2018 meta-analysis including a total of 96,595 patients concluded that NAFLD did increase the risk of incident chronic kidney disease with a hazard ratio of 1.37. Multiple confounding factors including age, sex, body mass index, serum lipids, hypertension, tobacco use, baseline kidney function, and diabetes were assessed and the association persisted. Statistical analysis confirmed that the risk of developing chronic kidney disease increased as NAFLD advanced [65]. Cross-sectional analysis showed a patient with liver fibrosis has a 2.5 times greater likelihood of having CKD and is twice as likely to have albuminuria then a patient without NAFLD [66]. It is hoped that medications that would target inflammation and fibrosis in nonalcoholic steatohepatitis (NASH) and chronic kidney disease may delay the disease progression of both these conditions.
OBSTRUCTIVE SLEEP APNEA
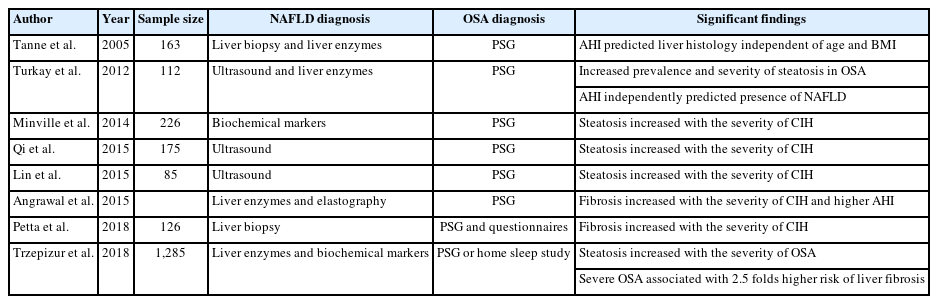
Most studies have concluded that a greater degree of hepatic steatosis increases the severity of chronic intermittent hypoxia on polysomnography (Table 1).
More concerningly, obstructive sleep apnea may contribute to the development of insulin resistance and it may trigger the development of nonalcoholic fatty liver disease [67]. Highlighting the need for multimodal therapy in NAFLD, chronic positive airway pressure treatment decreases the concentrations of liver enzymes, specifically alanine transaminase and aspartate transaminase [68].
ENDOCRINE CONDITIONS
Diabetes mellitus
The interest surrounding NAFLD and its predisposition to diabetes has been extensive. Patients with NAFLD generally have hepatic insulin resistance, which then increases the likelihood of developing diabetes mellitus. On a molecular level, it is thought that insulin resistance causes mitochondrial dysfunction which disrupts fatty acid beta oxidation and leads to lipid deposition in the liver [69]. Addressing NAFLD early on would decrease incident diabetes mellitus and its myriad associated complications. Measures aimed at weight loss, limiting saturated fats in the diet, and becoming physically active all increase insulin sensitivity and decrease hepatic steatosis [70]. Medications used for diabetes mellitus like pioglitazone are among the first line agents in the medical management of NASH [71].
Polycystic ovarian syndrome
The hallmark feature of polycystic ovarian syndrome (PCOS), androgen excess, has been related to insulin resistance. It is well recognized that higher rates of diabetes, central obesity, and dyslipidemia are observed in patients with PCOS [72]. NAFLD has also been shown to affect 34 to 70% of women with PCOS, when NAFLD affects only 14 to 34% of women in the general population [73].
Hyperandrogenism may independently increase the risk of NAFLD. A case-control study compared 275 non-obese women with PCOS to 892 non-obese women without PCOS. The PCOS cohort was found to have a NAFLD prevalence of 5.8%, while only 2.8% of women without PCOS had NAFLD. The study found that increased levels of free testosterone correlated to a higher risk for NAFLD even after adjusting for age, body-mass index, insulin resistance, and lipid profile [74].
Specific treatment of PCOS has not been shown to improve NAFLD. A common treatment for PCOS, oral contraceptives, have not had a clear benefit in NAFLD. A cross-sectional study looking at NHANES data did find lower rates of NAFLD in women currently on oral contraceptives when compared to women who had used them in the past or had never used them [75]. A biopsy-based study, however, showed increased lobular inflammation, a histologic feature of NASH, in patients taking oral contraceptives [76].
Weight loss, on the other hand, appears to be a more surefire way to ameliorate both conditions. Liraglutide 1.8 mg daily led to decreased rates of NAFLD along with downtrends in hepatic fat fraction and visceral adipose tissue [77]. The increased availability of glucagon-like peptide-1 receptor agonists worldwide remains a goal of clinicians invested in public health.
Hypothyroidism
The exact pathophysiological mechanisms for the development of NAFLD in the presence of hypothyroidism are yet to be elucidated. However, the most accepted mechanism of action is that hepatic steatosis results from decreased serum levels of thyroid hormone (TH). The decrease in TH stimulates lipolysis from fat stores in white adipose tissue and from dietary fat sources (high-fat diets) to generate free fatty acids that enter the hepatic cells via protein transporters causing an induction of de novo lipogenesis (DNL). In addition, TH indirectly controls the transcriptional regulation of hepatic DNL by regulating the expression and activities of other transcription factors such as sterol associated with NAFLD through increased levels of thyroid stimulating hormone (TSH) whereby high levels of TSH stimulate lipogenesis in the liver causing hepatosteatosis [78]. Disturbingly, hypothyroidism has been found to be more common in those with NASH and NAFLD related hepatocellular carcinoma [79-82]. Currently, there are no additional treatments recommended for this condition [83].
Growth hormone deficiency
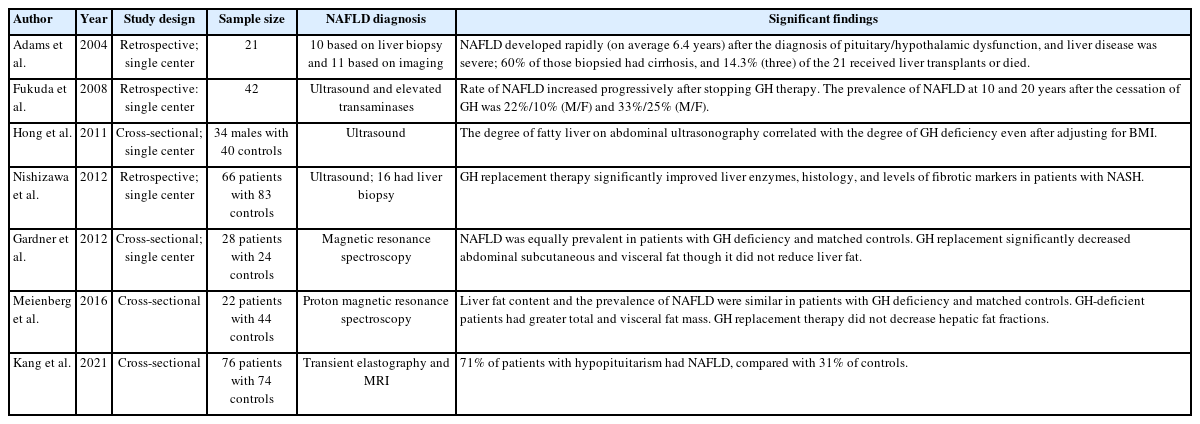
Ever since Takano et al published their case report of a 17 years old boy who presented with panhypopituitarism and fatty liver in 1997 [84], the therapeutic use of growth hormone (GH) in NAFLD has been explored by researchers around the world. In the case report, the patient was treated with GH and their fatty liver subsequently improved, as measured by ultrasound echogenicity and liver size. Numerous studies over the years looking at the relationship between NAFLD and GH deficiency are summarized in Table 2.
In patients with proven GH deficiency, replacing GH does decrease body fat content while increasing lean muscle mass [85]. Efforts at using GH in patients without GH deficiency with the aim of treating NAFLD have had mixed results so far. In a small pilot study, treatment with recombinant human growth hormone did not decrease liver fat content as assessed by magnetic resonance spectroscopy (MRS), though a lower body mass index was achieved [86].
NON-LIVER CANCER
Colon cancer
The links being found between NAFLD and cancer are alarming. Allen et al. [87] longitudinally followed a population of 4,722 patients with NAFLD and compared them to 14,441 controls and found that NAFLD doubled the risk of developing cancer while obesity alone did not (incidence rate ratio [IRR]=2.0, 95% confidence interval [CI] 1.5–2.9 vs. IRR=1.0, 95% CI 0.8–1.4). This data raises the concern that NAFLD may play a role in mediating cancer development. One theory proposes that visceral adipose tissue produces adipocytokines that lead to tumor proliferation. Gastrointestinal cancers appear to have the strongest correlation with NAFLD. Colon cancer specifically had an IRR of 1.8 in the study by Allen et al. [87]. A large meta-analysis of 15 studies confirms a similar degree of association, with a pooled OR of 1.7 when looking at NAFLD and the risk of colorectal cancer [88].
NAFLD appears to not only increase the risk of colon cancer, but precancerous lesions, as well. Adenomatous polyps, polyps with villous morphology, and lesions with high-grade dysplasia are all more common in patients with NAFLD [89]. The need for strict adherence to the recommended guidelines for colon cancer screening in patients with NAFLD are evident, though increased or earlier screening has not yet been suggested.
Gastric cancer
Another gastrointestinal cancer with links to NAFLD is stomach cancer. Data from six studies assessing the risk of incident stomach cancer in NAFLD showed a pooled random effects hazard ratio of 1.81 [90]. It is likely that similar pathways of tumorigenesis play a role in the development of these gastrointestinal cancers.
Breast cancer
The presence of NAFLD may also be associated with extragastrointestinal cancers. A pooled OR of 1.69 was found when assessing the risk of breast cancer in patients with NAFLD [88]. Some of the relevant studies are noted in Table 3.
Uterine cancer
Gynecologic cancers appear to be more prevalent in patients with NAFLD. In a pooled analysis of 85,827 patients, of which 23% had NAFLD, patients with NAFLD had an approximately 60% greater risk of developing uterine cancer than the general population [90].
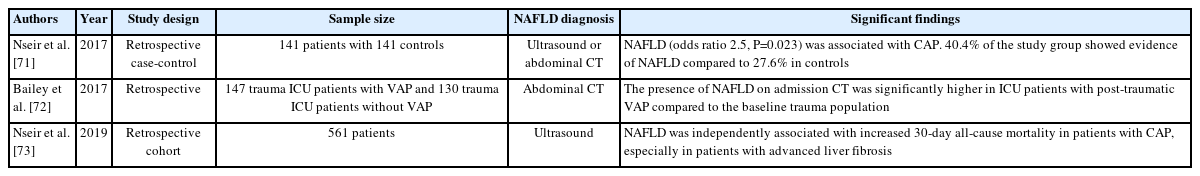
INFECTIONS
Helicobacter pylori
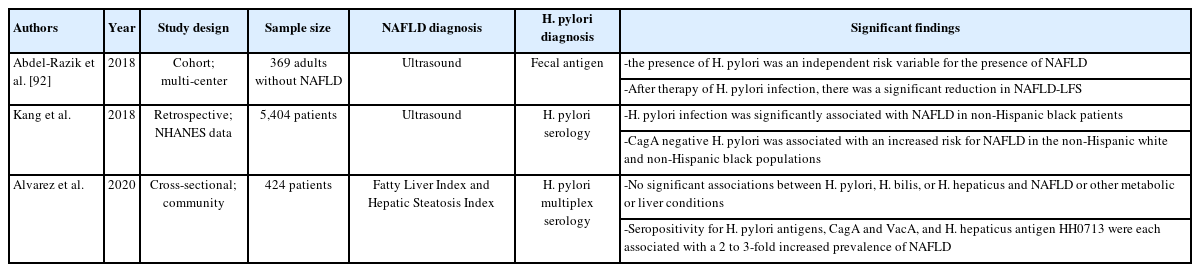
Gastrointestinal-specific infections have been associated with NAFLD. Helicobacter pylori increases the generation of inflammatory markers like interleukin-1β and tumor necrosis factor-α, levels of which are increased in patients testing positive for H. pylori. These markers may increase hepatic inflammation and predispose patients to developing NAFLD. Indeed, studies have shown a 36% greater risk of NAFLD in patients diagnosed with H. pylori infection [91] though the data does not universally affirm the risk (Table 4).
Data has been encouraging that treating H. pylori infection in patients with NAFLD does appear to improve fibrosis scores [92]. Clinicians should have a lower threshold for diagnosing and curing patients of H. pylori in cases of NAFLD.
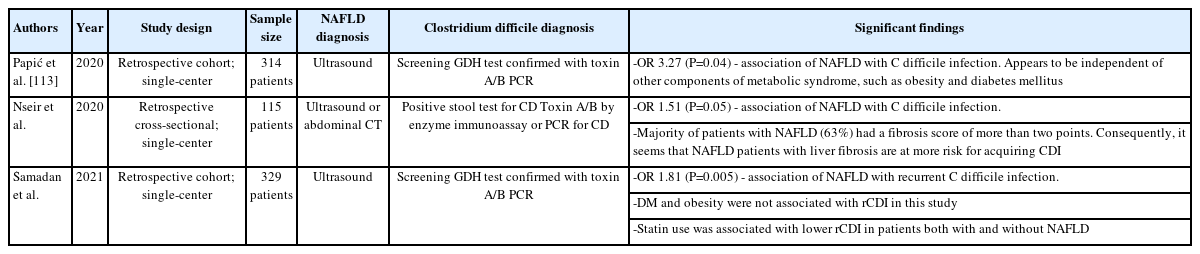
Clostridium difficile
Altered gut microbiome in patients with NAFLD is also being explored. Patients with NASH are found to have increased amounts of Bacteroides and decreased amounts of Prevotella in their gastrointestinal flora, while Ruminococcus was associated with increased liver fibrosis [93]. It follows that patients with NAFLD have a higher risk of infection with Clostridium difficile, even after adjusting for the presence of diabetes and obesity (Table 5).
COVID-19
During the global pandemic of our time, front-line clinicians early on saw the increased mortality rates among patients with obesity. NAFLD by itself appears to increase the risk further, even after adjusting for the presence of obesity, especially in severe COVID-19 disease [94]. NAFLD also increases the duration of viral shedding [95], a finding with public health implications. The liver is thought to be especially prone to this virus as SARS-COV-2 enters cells through the angiotensin-converting enzyme 2 (ACE2). These enzymes are abundant in both the liver and in the biliary epithelium [96]. Fighting NAFLD on all fronts may very well decrease the rapid spread of any future viral respiratory-borne infections.
OVERALL PATIENT REPORTED OUTCOMES (PROs)
The presence of NAFLD or NASH is associated with decreased PROs which is more evident in those with NASH and advanced fibrosis. Among the studies completed on health-related quality of life, results have consistently shown that patients with NAFLD and NASH report low physical functioning scores, fatigue and higher rates of depression and anxiety than the general population which can result in decreased productivity at work if employed (presenteeism) and/or performing their activities of daily living [97-100]. On the other hand, treatment of NAFLD or NASH that causes a regression in the disease state patients may show an improvement in their PROs.
Health care utilization for both inpatient and outpatient care is increased for those with NAFLD especially when the comorbidities of CVD, hypertension, and obesity were present for inpatients and CVD, diabetes mellitus, hypertension were present as outpatients. However, the presence of cirrhosis increased costs significantly among inpatients and outpatients. In addition, NAFLD has a significant economic impact on countries, as well [101-113].
CONCLUSION
NAFLD is a complex metabolically based liver disease that is associated with a number of comorbidities. Through an increased awareness of the extrahepatic complications of NAFLD, clinicians can embark on a multi-pronged approach to tackle this insidious, mostly asymptomatic condition [27-34]. As more research is completed on finding patients with NAFLD who are at the highest risk for adverse outcomes, further study is required to determine the preventative screening guidelines to be implemented due to their demonstrably greater risk in several conditions. Due to its multifaceted nature, effective treatments of NAFLD may be generated in other fields not directly related to hepatology, and these developments will be followed with interest by hepatologists worldwide.
Notes
Authors’ contribution
RM: study design, data collection, data synthesis and interpretation, and drafting of the manuscript. MHN: study concept, study supervision, data interpretation, and revision of the manuscript. All authors approved the final draft of the manuscript as well as the authorship list.
Conflicts of Interest
Mindie H. Nguyen: Research support: Pfizer, Enanta, Gilead, Glycotest, Vir, B.K. Kee Foundation, National Cancer Institute. Advisory board/consulting: Janssen, Spring Bank, Gilead, Novartis, Bayer, Eisai, Eli Lilly, Exact Sciences, Laboratory of Advanced Medicine, Helio Health, Intercept. Other authors have no disclosures.
Abbreviations
NAFLD
nonalcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
CVD
cardiovascular disease
HFpEF
heart failure with preserved ejection fraction
HFrEF
heart failure with reduced ejection fraction
AVS
aortic valve sclerosis
MAC
mitral annular calcification
OR
odds ratio
CIMT
carotid intima-media thickness
AST
aspartate transaminase
ALT
alanine transaminase
GGT
gamma-glutamyltransferase
HMG-CoA reductase
hydroxy-methyl-glutaryl-coenzyme A reductase
PCSK 9 and 7
proprotein convertase subtilisin/kexin type 9 and 7
CKD
chronic kidney disease
OSA
obstructive sleep apnea
BMI
body mass index
PSG
polysomnography
AHI
apnea-hypopnea index
CIH
chronic intermittent hypoxia
GH
growth hormone
BMI
body mass index
M/F
males/females
US
ultrasound
MRI
magnetic resonance imaging
CT
computerized tomography
DM
diabetes mellitus
PCOS
polycystic ovarian syndrome
OCP
oral contraceptive pills
LDL
low-density lipoprotein
GLP- 1
glucagon-like peptide-1
TH
thyroid hormones
TSH
thyroid stimulating hormone
rhGH
recombinant human growth hormone
ICD-9
International Classification of Diseases
H. pylori
Helicobacter pylori
LFS
liver fibrosis score
CagA
cytotoxin associated gene A
VacA
vacuolating cytotoxin A
CAP
communityacquired pneumonia
VAP
ventilator-associated pneumonia
CT
computerized tomography
ICU
intensive care unit
C. difficile and CD
Clostridium difficile
rCDI
recurrent C. difficile infection
GDH
glumatate dehydrogenase
PCR
polymerase chain reaction
ACE2
angiotensin-converting enzyme 2
COVID-19
coronavirus disease 2019
SARS-COV-2
severe acute respiratory syndrome coronavirus 2
PROs
patient-reported outcomes