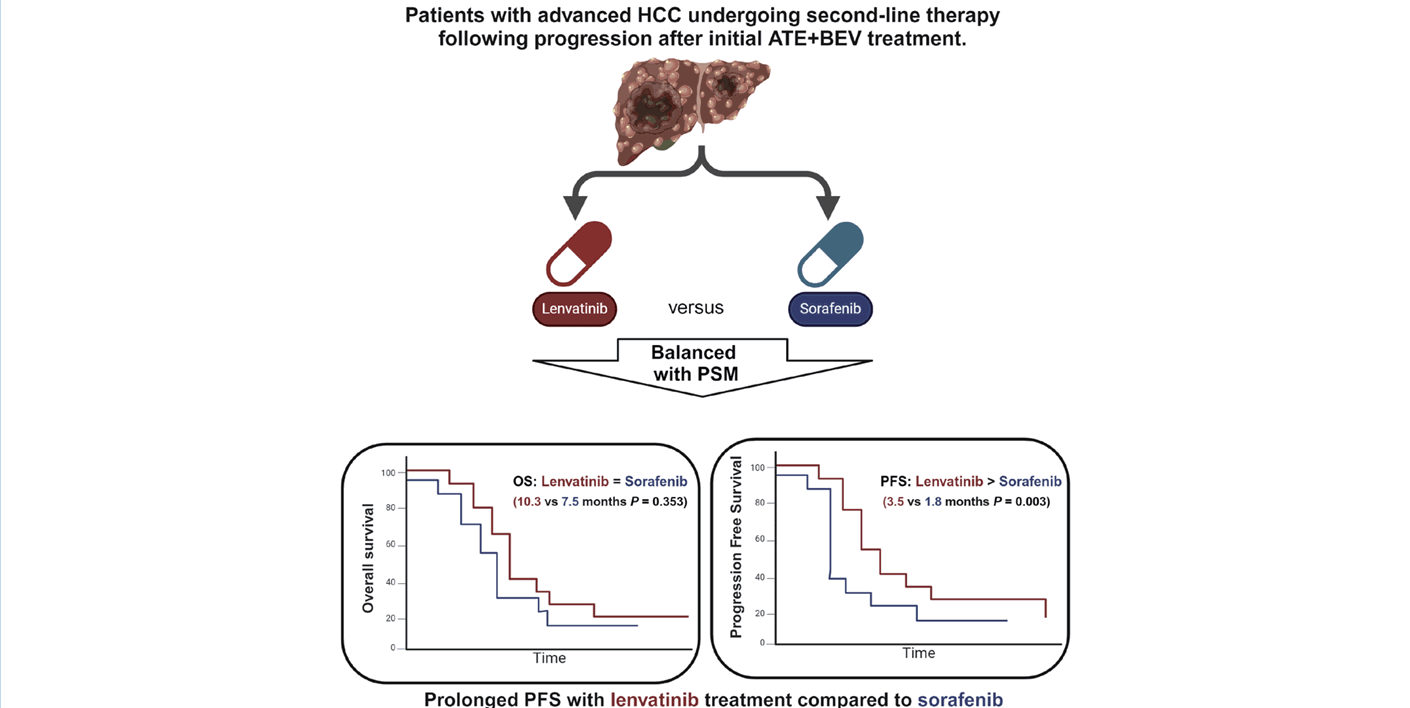
Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study
-
Young Eun Chon1,*, Dong Yun Kim2,*, Mina Kim2, Beom Kyung Kim2, Seung Up Kim2, Jun Yong Park2, Sang Hoon Ahn2, Yeonjung Ha1, Joo Ho Lee1, Kwan Sik Lee1, Beodeul Kang3, Jung Sun Kim3, Hong Jae Chon3
 , Do Young Kim2
, Do Young Kim2
- Received December 21, 2023 Revised March 1, 2024 Accepted March 8, 2024
- ABSTRACT
-
- Background/Aims
- Atezolizumab plus bevacizumab (ATE+BEV) therapy has become the recommended first-line therapy for patients with unresectable hepatocellular carcinoma (HCC) because of favorable treatment responses. However, there is a lack of data on sequential regimens after ATE+BEV treatment failure. We aimed to investigate the clinical outcomes of patients with advanced HCC who received subsequent systemic therapy for disease progression after ATE+BEV.
- Methods
- This multicenter, retrospective study included patients who started second-line systemic treatment with sorafenib or lenvatinib after HCC progressed on ATE+BEV between August 2019 and December 2022. Treatment response was assessed using the Response Evaluation Criteria in Solid Tumors (version 1.1.). Clinical features of the two groups were balanced through propensity score (PS) matching.
- Results
- This study enrolled 126 patients, 40 (31.7%) in the lenvatinib group, and 86 (68.3%) in the sorafenib group. The median age was 63 years, and males were predominant (88.1%). In PS-matched cohorts (36 patients in each group), the objective response rate was similar between the lenvatinib- and sorafenib-treated groups (5.6% vs. 8.3%; P=0.643), but the disease control rate was superior in the lenvatinib group (66.7% vs. 22.2%; P<0.001). Despite the superior progression-free survival (PFS) in the lenvatinib group (3.5 vs. 1.8 months, P=0.001), the overall survival (OS, 10.3 vs. 7.5 months, P=0.353) did not differ between the two PS-matched treatment groups.
- Conclusions
- In second-line therapy for unresectable HCC after ATE+BEV failure, lenvatinib showed better PFS and comparable OS to sorafenib in a real-world setting. Future studies with larger sample sizes and longer follow-ups are needed to optimize second-line treatment.
- INTRODUCTION
- INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the second leading cause of cancer deaths in South Korea and worldwide [1,2]. In contrast to patients with early-stage or intermediate-stage HCC, who may have many treatment options for HCC such as surgery, radiofrequency ablation, transplantation, or trans-arterial chemoembolization, patients with advanced HCC typically only derive survival benefits from systemic therapies [3,4]. Unfortunately, more than 50% of patients with HCC are first diagnosed at advanced stages, and these patients are more likely to undergo systemic therapies.Sorafenib, an oral tyrosine kinase inhibitor (TKI), has been used as a standard systemic HCC treatment since 2007 [5,6]. However, another potent TKI lenvatinib was introduced in 2018 and has played a leading role in improving the clinical outcomes of advanced HCC [7]. In 2020, an immunotherapy-based combination regimen of atezolizumab (ATE, an immune checkpoint inhibitor) and bevacizumab (BEV, a targeted therapy agent) provided a survival benefits over sorafenib in overall survival (OS) at 12 months (67.2% vs. 54.6%, P<0.001) and progression-free survival (PFS, 6.8 vs. 4.3 months, P<0.001) in patients with advanced HCC [8]. Currently, the landscape of systemic treatment for unresectable HCC has changed to the use of ATE+BEV as the initial treatment unless there are contraindications.However, the optimal second-line and subsequent treatments for patients with advanced HCC who have progressed on first-line ATE+BEV treatment have not been clearly defined in the national and international guidelines for HCC [9,10]. This may be because the molecular expression of HCC is so diverse that there are no established biomarkers to guide subsequent treatment, and survival and clinical outcomes have not been established in real-world settings [11].Only a few clinical studies have demonstrated the OS and PFS of patients who underwent various second-line therapies such as lenvatinib or sorafenib after ATE+BEV failure. However, there was an insufficient number of patients in these studies, and baseline characteristics were not adjusted for an exact comparison [12-15]. In the present study, we compared the clinical effectiveness of sorafenib and lenvatinib as a second-line treatment after failure of ATE+BEV in unadjusted and matched patient cohorts with advanced HCC in a real-world setting.
- MATERIALS AND METHODS
- MATERIALS AND METHODS
- Patients and definitions
- Patients and definitions
This retrospective study included patients who started second-line systemic treatment with sorafenib or lenvatinib after HCC progressed on ATE+BEV between August 2019 and December 2022 at one of two university hospitals (Severance Hospital and CHA Bundang Medical Center). All study patients had been diagnosed with unresectable HCC, either histologically or clinically, following HCC guidelines [16].Recorded data included age, sex, performance status and serum concentrations of tumor markers of alpha-fetoprotein (AFP) and protein induced by vitamin K antagonist-II (PIVKA-II). Hepatitis B virus (HBV) infection was defined as hepatitis B surface antigen seropositivity for more than 6 months, and hepatitis C virus (HCV) infection was defined as seropositivity for anti-HCV antibody. HBV and/or HCV infection were classified as viral etiology, whereas alcohol, non-alcoholic steatohepatitis, and other chronic liver diseases were classified as non-viral etiologies. The hepatic functional reserve was assessed with the Child-Pugh score. Tumor characteristics assessed in all patients included Barcelona Clinic Liver Cancer (BCLC) stage; tumor number; tumor size; and the presence of lymph node metastasis, extrahepatic metastasis, macrovascular invasion, and extrahepatic lesions. The number of previous ATE+BEV treatment cycles was also recorded. The study protocol was reviewed and approved by the Institutional Review Boards of Severance Hospital (IRB No. #4-2023-1273) and CHA Bundang Medical Center (IRB No. #2021-07-071), which waived the requirement for informed patient consent due to the retrospective nature of the analyses. The study was performed in accordance with the ethical standards of the latest amended Declaration of Helsinki.- Treatment regimens
- Treatment regimens
All study patients had been treated previously with a combination of ATE (1,200 mg) and BEV (15 mg/kg), administered intravenously every 3 weeks. After confirmation of tumor progression on ATE+BEV treatment, subsequent systemic therapy was administered at the discretion of the attending specialists. The dose and interval of treatment regimen adhered to standard protocols [5,7]. Patients received oral lenvatinib (12 mg/day for bodyweight ≥60 kg or 8 mg/day for bodyweight <60 kg) or sorafenib 400 mg twice-daily for 4 weeks in a cycle. Treatment was continued until disease progression, unacceptable toxicity, or death. Dosage was adjusted considering each patient’s tolerability.- Assessment of clinical outcomes
- Assessment of clinical outcomes
Patients received oral lenvatinib or sorafenib for 4 weeks per cycle, with treatment response and safety evaluated every 8 to 12 weeks (i.e., after every two to three treatment cycles). The radiological response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 based on the results of liver dynamic computed tomography or magnetic resonance imaging (if appropriate) [16,17]. The objective response rate (ORR) was defined as the proportion of patients who achieved a complete response (CR) or partial response (PR), and the disease control rate (DCR) was defined as the proportion of patients who achieved CR, PR, or stable disease (SD). Treatment-related adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events version 5.0. OS was defined as the interval from the initiation of second-line treatment to death or final follow-up, and PFS was defined as the interval from the initiation of second-line treatment to the date of disease progression or death from any cause, whichever occurred first.- Statistical analysis
- Statistical analysis
Variables were expressed as mean ± standard deviation, median (interquartile range [IQR]), and number (%). Differences between continuous variables were assessed by Student’s t-tests or Mann–Whitney U-tests, whereas differences between categorical variables were assessed by chi-square tests or Fisher’s exact tests. Survival curves were generated by the Kaplan–Meier method and differences between treatment groups were assessed by log-rank tests. Factors independently predictive of OS and PFS were assessed using a multivariable Cox proportional hazards regression model. The possible effects of selection bias and potential confounders between the two groups were reduced by 1:1 propensity score (PS) matching. Statistical analyses were performed using SAS software (ver. 9.4; SAS Institute, Cary, NC, USA) and R software (version 4.3.1; http://cran.r-project.org/, accessed 16 June 2023). Two-sided P-values <0.05 were considered statistically significant.
- RESULTS
- RESULTS
- Baseline characteristics of the study population
- Baseline characteristics of the study population
Figure 1 shows a diagram of the study population. A total of 194 patients who received ATE+BEV as first-line systemic therapy and subsequently discontinued treatment between August 2019 and December 2022 at one of two university hospitals (Severance Hospital and CHA Bundang Medical Center) was considered eligible. During ATE+BEV treatment, 34 patients died, and 8 patients experienced compromised liver function and/or clinical deterioration. Thus, excluding these 42 patients, 152 patients discontinued ATE+BEV due to disease progression. After excluding 16 more patients who experienced compromised liver function and/or clinical deterioration after cessation of ATE+BEV, a total of 136 patients received second-line treatment after progression on first-line ATE+BEV treatment. Of these patients, 86 (63.2%) received sorafenib, 40 (29.4%) received lenvatinib, 8 (5.9%) received regorafenib, and 2 (1.5%) underwent 5-FU based infusion chemotherapy. Finally, the present study enrolled 126 patients, 40 (31.7%) who received lenvatinib, and 86 (68.3%) who received sorafenib after ATE+BEV failure.The baseline characteristics of the patients at the start of second-line treatment are depicted in Table 1. The median patient age was 63 years (IQR, 55–70 years), and 111 (88.1%) patients were male. HCC was of viral etiology in 92 (73.0%) patients, 109 (86.6%) patients were classified as having BCLC stage C tumors, and 91 (72.2%) as having Child-Pugh A liver function. The median number of previous ATE+BEV treatment cycles was 4 (IQR, 3–6). The proportion of patients with Child-Pugh A liver function was significantly higher (92.5% vs. 62.8%, P=0.001) and the median number of previous ATE+BEV treatment cycles was significantly higher in the lenvatinib group compared to the sorafenib group (6 [IQR, 4–10] vs. 3 [IQR, 2–6], P<0.001). There were no other significant differences in baseline characteristics between the 2 groups.To reduce the effects of confounding variables and selection bias, PS matching was conducted using the following factors: age, sex, tumor size, tumor number, extrahepatic metastasis, lymph node metastasis, and Child-Pugh class. PS matching on a 1:1 ratio resulted in 36 pairs of patients with balanced baseline characteristics. However, the median number of ATE+BEV treatment cycles was significantly higher in the lenvatinib group than in the sorafenib group (Table 1).- Clinical responses of second-line treatment
- Clinical responses of second-line treatment
Responses to second-line treatment according to RECIST 1.1 and mRECIST criteria are presented in Table 2. None of the patients in either group achieved CR, whereas 3 (7.5%) patients in the lenvatinib group and 5 (5.8%) in the sorafenib group achieved PR. ORR was similar between the lenvatinib and sorafenib groups(7.5% vs. 5.8%, P=0.719). As SD was significantly higher in the lenvatinib group (60.0% vs. 18.6%, P<0.001), DCR was significantly higher in the lenvatinib than in the sorafenib group (67.5% vs. 24.4%; P<0.001). Treatment responses in the PS-matched cohort were similar to those in the unadjusted cohort, showing significantly higher DCR in the lenvatinib group compared to the sorafenib group (66.7% vs. 22.2%, P<0.001, Table 2). Assessment of treatment responses according to mRECIST criteria yielded comparable results, with ORRs not differing significantly between the two treatment groups and DCR being significantly higher in the lenvatinib group (P<0.001).- Survival outcomes of second-line treatment
- Survival outcomes of second-line treatment
In the unadjusted cohort, 81 (64.3%) patients died after a median follow-up of 5.5 months (IQR, 3.5–9.3 months), and 107 (83.6%) patients experienced disease progression after a median follow-up of 2.1 months(IQR, 1.4–3.5 months). Evaluation of the unadjusted cohort showed significantly longer median OS in the lenvatinib group than in the sorafenib group (10.3 [IQR, 6.8–N/A] vs. 5.6 [IQR, 4.7–9.0] months, P=0.019; Fig. 2A, Table 3). Median PFS was also significantly longer in the lenvatinib than in the sorafenib group (3.5 [IQR, 3.0–4.2] vs. 1.8 [IQR, 1.6–2.3] months, P=0.001; Fig. 2B, Table 3). In the PS-matched cohort, 44 (61.1%) patients died after a median follow-up of 8.0 months (IQR, 5.7–10.9 months), and 61 (84.7%) patients experienced disease progression after a median follow-up of 2.8 months(IQR, 1.9–3.5 months).Following PS matching, however, the median OS did not differ between the two groups (lenvatinib vs. sorafenib, 10.3 [IQR, 6.1–N/A] vs. 7.5 [IQR, 4.7–11.3] months, P=0.353; Fig. 3A, Table 3). PFS showed a similar trend to the unadjusted cohort of and was superior PFS in the lenvatinib group even after PS matching (lenvatinib vs. sorafenib, 3.5 [IQR, 3.0–4.2] vs. 1.8 [IQR, 1.6–2.4] months, P=0.003; Fig. 3B, Table 3).- Predictors for survival outcomes
- Predictors for survival outcomes
Table 4 depicts the predictors for survival outcomes. During the median follow-up of 5.5 (IQR, 3.47–9.33) months, 81 (64.3%) patients died. Univariable Cox regression analyses showed ECOG performance score 2, Child-Pugh class B, tumor size ≥10 cm, PIVKA-II concentration ≥1,000 mAU/mL, sorafenib treatment, and number of previous ATE+BEV treatment cycles (<3) as predictors for death. Subsequent multivariable analyses revealed that Child-Pugh class B (adjusted hazard ratio [aHR]=2.472; 95% confidence interval [CI], 1.433–4.266; P=0.001), and PIVKA-II concentration ≥1,000 mAU/mL (aHR=1.710; 95% CI, 1.015–2.883; P=0.044) were independently predictive of death.Over a median follow-up of 2.07 (IQR, 1.37–3.47) months, 107 (83.6%) patients showed disease progression. Univariable Cox-regression analyses selected ECOG performance score 2, Child-Pugh class B, extrahepatic metastasis,sorafenib treatment, and number of previous ATE+BEV treatment cycles (<3) as significant predictors for disease progression. Subsequent multivariable analyses showed extrahepatic metastasis (aHR=1.991; 95% CI, 1.265–3.132; P=0.002) and sorafenib treatment (aHR=1.852; 95% CI, 1.142–3.003; P=0.012) to be independent predictors for disease progression.- Survival outcomes according to third-line treatment
- Survival outcomes according to third-line treatment
Eighteen (40%) patients in the lenvatinib group and 44 (51.2%) patients in the sorafenib group received third-line treatment (P=0.519). The third-line treatment options included cabozantinib, ipilimumab plus nivolumab, nivolumab, regorafenib, and sorafenib (Supplementary Table 1). In the lenvatinib group, the median OS did not differ between patients who did and did not receive third-line treatment in both the unmatched (P=0.870, Supplementary Fig. 1A) and PS-matched (P=0.940, Supplementary Fig. 1B) cohorts. However, median OS was significantly affected by the type of third-line treatment in both the unmatched (P<0.001, Supplementary Fig. 2A) and PS-matched (P=0.002, Supplementary Fig. 2B) cohorts. Most of the patients (15/18, 83.3%) in the lenvatinib group received sorafenib as third-line treatment, with few or none receiving other third-line treatments.Evaluation of the sorafenib group showed that OS was significantly longer among patients in the unmatched cohort who did than did not receive third-line treatment (P=0.009, Supplementary Fig. 1C), although this trend was not observed in the PS-matched cohort (P=0.440, Supplementary Fig. 1D). Median OS was not affected by the type of third-line treatment in both the unmatched (P=0.920, Supplementary Fig. 2C) and PS-matched (P=0.330, Supplementary Fig. 2D) cohorts.- Survival outcomes according to number of previous ATE+BEV treatment cycles
- Survival outcomes according to number of previous ATE+BEV treatment cycles
There was no difference in survival outcomes between the lenvatinib group and sorafenib group according to the number of previous ATE+BEV treatment cycles (≤3 vs. >3) in both the unmatched and PS-matched cohorts (Supplementary Figs. 3, 4 and Supplementary Table 2).- Safety profiles
- Safety profiles
Treatment-related AEs are shown in Table 5. Treatment-related AEs of any grade occurred in 32 patients (80.0%) of the lenvatinib group and 75 patients (87.2%) of the sorafenib group (P=0.282). Grade 3or 4 AEs occurred in 35.0% and 38.4% of patients in the lenvatinib and sorafenib groups, respectively (P=0.145). There were no deaths caused directly by grade 5 AEs. Most AEs were manageable, and the percentage of AEs leading to the discontinuation of treatment did not differ between the two groups (lenvatinib vs. sorafenib, 12 [30.0%] vs. 16 [18.6 %] patients; P=0.152).The most common AEs of any grade in the lenvatinib group were proteinuria (57.5%), AST elevation (50.0%), and thrombocytopenia (50.0%), and while those in the sorafenib group were total bilirubin elevation (54.7%), AST elevation (52.3%), and ALT elevation (25.6%). The most common grade 3 or 4 AEs in the lenvatinib group were proteinuria (30.0%), AST elevation (10.0%), and gastrointestinal bleeding (7.5%), and those in the sorafenib group were rash (4.7%), hand-foot syndrome (HFS, 4.7%), and AST elevation (2.3%).HFS was significantly more frequent in the sorafenib group than in the lenvatinib group (19.8% vs. 5.0%, P=0.031). In contrast, rates of hypertension, thrombocytopenia, anemia, anorexia, proteinuria, and hypothyroidism (all P<0.05) were significantly higher in the lenvatinib group than in the sorafenib group.
- DISCUSSION
- DISCUSSION
One of the major strengths of this study was its comparison of treatment outcomes and survival in matched cohorts of patients with HCC who received second-line lenvatinib or sorafenib who experienced progression on ATE+BEV treatment. This is the first study to compare treatment outcomes and survival of second-line lenvatinib and sorafenib in matched HCC patient cohorts who experienced progression after ATE+BEV treatment. PFS was significantly superior in the lenvatinib group in both unadjusted and matched cohorts. However, OS seemed to be longer in the lenvatinib group in the original cohort but was not statistically different after PS matching between the 2 treatment groups.The main survival outcomes of our study are in line with a previous study by Yoo et al. [13]. In that study, the median PFS was significantly higher in the lenvatinib group compared to the sorafenib group (6.1 vs. 2.5 months, P=0.004), whereas the median OS was not significantly different between the 2 groups (16.6 vs. 11.2 months, P=0.347). The results of our study reinforce the previous results by reproducing the same trend even after adjusting for baseline characteristics by PS matching. A recent global study by Persano et al. [18] showed significantly better OS and PFS in the lenvatinib group compared to the sorafenib group (hazard ratio for OS, 0.45; 95% CI, 0.24–0.83; reference sorafenib group). The superior PFS may partially attributed to the inherent potential of lenvatinib which slows disease progression and prolongs PFS. When lenvatinib was used as a first-line systemic treatment in the REFLECT trial [7], it showed significantly better PFS than sorafenib. In our previous study comparing the efficacy between first-line ATE+BEV and lenvatinib, lenvatinib showed non-inferior PFS compared to ATE+BEV [19]. In a Japanese single-arm study showing oncologic outcomes of lenvatinib after ATE+BEV failure [20], the median OS and PFS were comparable to those of Yoo et al. [13] (OS, 15.7 months in Japanese study vs. 16.6 months in Yoo et al. [13]; PFS, 4.4 vs. 6.1 months). Another Japanese study reported that the median OS and PFS of patients treated with lenvatinib after ATE+BEV failure were 13.5 months and 4.0 months, respectively, similar to previous findings [21]. Median OS and PFS were slightly shorter, at 12.8 months and 3.7 months, respectively, in a US study that included mostly Caucasian patients [22]. In our multivariate analyses of predictors for survival outcome, lenvatinib was a significant predictor for better PFS. These collective findings support the superiority of lenvatinib over sorafenib on PFS after ATE+BEV treatment failure. However, as several other clinical trials (regorafenib, cabozantinib) are investigating the efficacy of second-line TKIs with different targets in patients with HCC after ATE+BEV failure, a comparison of results between these drugs is anticipated [23,24].The relatively low ORR of lenvatinib in second-line treatment may contribute to the comparable OS between the 2 groups. According to the REFLECT trial, which compared oncologic and survival outcomes between lenvatinib and sorafenib as a first-line treatment in patients with HCC, the ORR of lenvatinib was much higher than that of sorafenib when assessed with mRECIST (40% vs. 13%) and RECIST 1.1 (19% vs. 7%) [7]. However, the ORR of the second-line treatments in our study was 7.5% in the lenvatinib group and 5.8% in the sorafenib group. In PS-matched analysis, the ORR was reversed, and sorafenib showed a numerically better rate over lenvatinib (5.6% vs. 8.3%) but without statistical significance. Furtherstudies are needed to determine whether the ORR of lenvatinib may be reduced by prior exposure to an anti-angiogenic agent such as BEV.Another peculiar finding of our study is that survival outcomes of both drugs were inferior to those of Yoo et al. [13] (lenvatinib OS, 16.6 months; sorafenib OS, 11.2 months) and those of Persano et al. [18] (lenvatinib OS, 17.0 months;sorafenib OS, 14.2 months). These differences could have occurred because our study population included different baseline characteristics and larger numbers of Child-Pugh class B patients with poor liver function and ECOG 2 patients with poor performance. These findings are in agreement with results showing that PFS and OS were longer in patients with Child-Pugh Class A than Class B liver function who were treated with lenvatinib after ATE+BEV failure [22]. Since study by Yoo et al. [13] only included patients who received ATE+BEV in clinical trials, patients in that study are more selected ones compared to those from real-world study. The proportion of patients (89.5%, 136/152) who received second-line treatment after disease progression instead of conservative care after disease progression on ATE+BEV was higher in our study than in other studies [13,14,18]. This implies that more patients with marginal liver function or poor general condition after first-line treatment underwent second-line treatment.In this study, the absence of extrahepatic metastasis at the start of second-line treatment and receipt of lenvatinib treatment were significant predictors for better PFS, and low PIVKA-II level and good liver function were associated with superior OS. Aside from known predictors such as liver function or tumor extent, there is great interest in the choice of individual-based secondary drug selection for effects on clinical outcomes. There is an ongoing concern about whether to rechallenge with immune treatment or to use TKI after ATE+BEV failure. For patients who have achieved a durable response to ATE+BEV for a long time, some suggest that another immunotherapy-based regimen would be beneficial than switching to a TKI [25]. In our subgroups analyses, neither third-line treatment or previous numbers of ATE+BEV cycle differentiate the OS of the patient. Since the analysis related to third-line treatment was conducted on a limited sample size, further verification in a larger cohort is necessary to confirm these findings. In addition, future studies should focus on finding biomarkers to determine whether to continue immunotherapy or to switch to targeted therapy.This study had several limitations. First, the study design was retrospective, which may have introduced selection bias and confounders. We adopted various statistical methods to overcome these limitations by performing PS matching and multivariate regression analyses to adjust for different baseline characteristics. Second, we only included Asian patients from 2 centers, and it was difficult to conduct a multifaceted analysis; thus, the conclusion may not be generalized to patients of other races and countries. Third, as ATE+BEV was introduced in Korea in May 2020, the follow-up duration for second-line treatment may be insufficient to draw firm conclusion. Despite these limitations, this is the first study to suggest the oncologic outcomes of patients who underwent second-line lenvatinib or sorafenib treatment after ATE+BEV treatment failure, with adjustments of baseline characteristics by PS matching.In conclusion, lenvatinib showed favorable PFS with similar OS compared to sorafenib as a second-line therapy for unresectable HCC after ATE+BEV failure in a well-matched cohort and real-world setting. Future studies with a larger sample size and longer follow-up are needed to confirm this finding and to optimize second-line treatment in such patients.
- FOOTNOTES
- FOOTNOTES
-
Authors’ contribution Conception: Young Eun Chon, Dong Yun Kim, Hong Jae Chon, and Do Young Kim; study design: Young Eun Chon, Dong Yun Kim, Hong Jae Chon, and Do Young Kim; participation in patient management and data collection: Young Eun Chon, Dong Yun Kim, Hong Jae Chon, and Do Young Kim; contribution to data acquisition: Young Eun Chon, Dong Yun Kim, Mina Kim, Beom Kyung Kim, Jun Yong Park, Yeonjung Ha,Joo Ho Lee, and Kwan Sik Lee, writing the paper: Young Eun Chon, Dong Yun Kim, Hong Jae Chon, and Do Young Kim; statistical analysis: Young Eun Chon, Dong Yun Kim, Hong Jae Chon, and Do Young Kim. All authors have reviewed the paper and approved the final version.
Conflicts of Interest HJ Chon has received honoraria from Eisai, Roche, Bayer, ONO, MSD, BMS, Celgene, Sanofi, Servier, AstraZeneca, Sillajen, Menarini, GreenCross Cell, Boryung Pharmaceuticals, and Dong-A ST, and has received research grants from Roche, Dong-A ST, and Boryung Pharmaceuticals. The other authors have no potential conflicts of interest to disclose.
SUPPLEMENTAL MATERIAL
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).Supplementary Table 1.
cmh-2023-0553-Supplementary-Table-1.pdfOverview of distribution and survival outcomes based on types of third-line treatmentsSupplementary Table 2.
cmh-2023-0553-Supplementary-Table-2.pdfPatient survival outcomes based on the duration of ATE+BEV treatmentSupplementary Figure 1.
cmh-2023-0553-Supplementary-Fig-1.pdfKaplan–Meier analysis of overall survival depending on administration of third-line treatment. (A) Lenvatinib-unmatched patients. (B) Lenvatinib-PS-matched patients. (C) Sorafenib-unmatched patients. (D) Sorafenib-PS-matched patients.Supplementary Figure 2.
cmh-2023-0553-Supplementary-Fig-2.pdfKaplan–Meier analysis of overall survival depending on the types of third-line treatment. (A) Lenvatinib-unmatched patients. (B) Lenvatinib-PS-matched patients. (C) Sorafenib-unmatched patients. (D) Sorafenib-PS-matched patients.Supplementary Figure 3.
cmh-2023-0553-Supplementary-Fig-3.pdfKaplan–Meier survival analysis comparing different durations of ATE+BEV treatment in overall patients. (A, B) Survival outcomes for patients treated with ATE+BEV for 3 cycles or fewer. (A) Overall survival; (B) Progression-free survival. (C, D) Survival outcomes for patients treated with ATE+BEV for more than 3 cycles. (C) Overall survival; (D) Progression-free survival. ATE+BEV, atezolizumab plus bevacizumab; PFS, progression-free survival; OS, overall survival.Supplementary Figure 4.
cmh-2023-0553-Supplementary-Fig-4.pdfKaplan–Meier survival analysis comparing different durations of ATE+BEV treatment in PS patients. (A, B) Survival outcomes for patients treated with ATE+BEV for over than 3 cycles. (A) Overall survival; (B) Progression-free survival. (C, D) Survival outcomes for patients treated with ATE+BEV for more than 3 cycles. (C) Overall survival; (D) Progression-free survival. ATE+BEV, atezolizumab plus bevacizumab; PFS, progression-free survival; OS, overall survival.
Figure 1.
Figure 2.
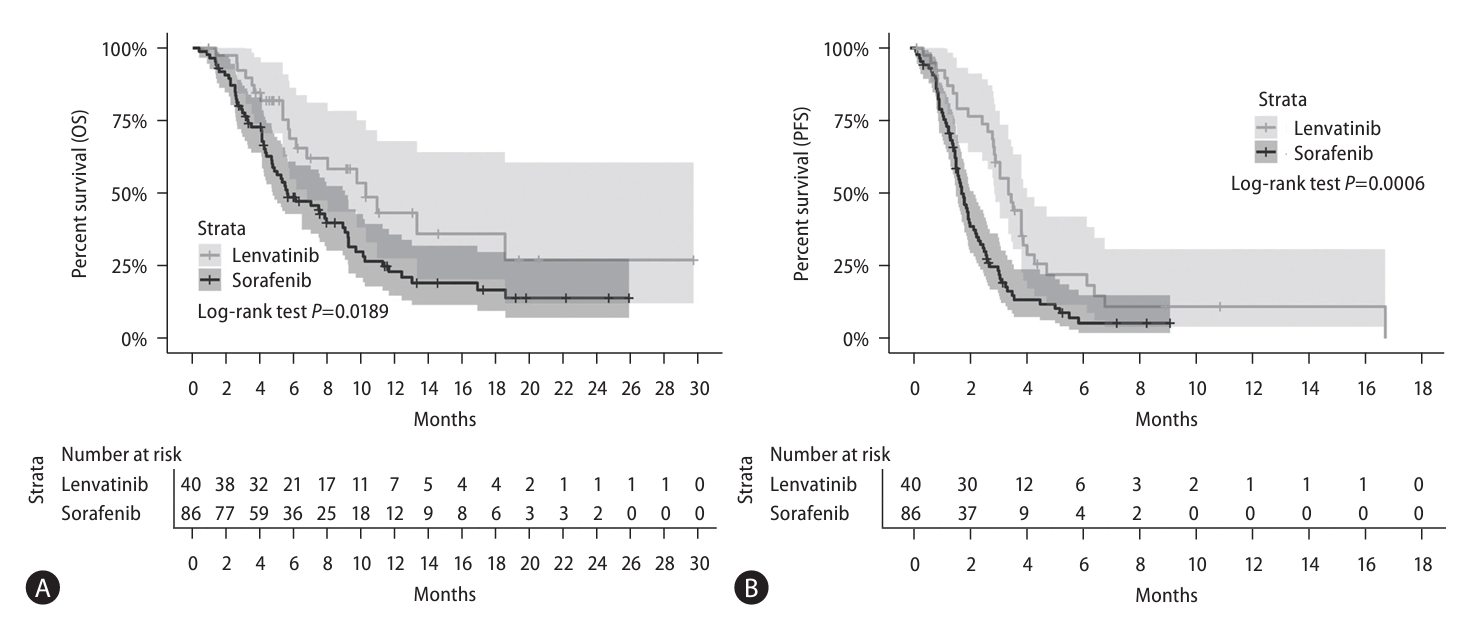
Figure 3.
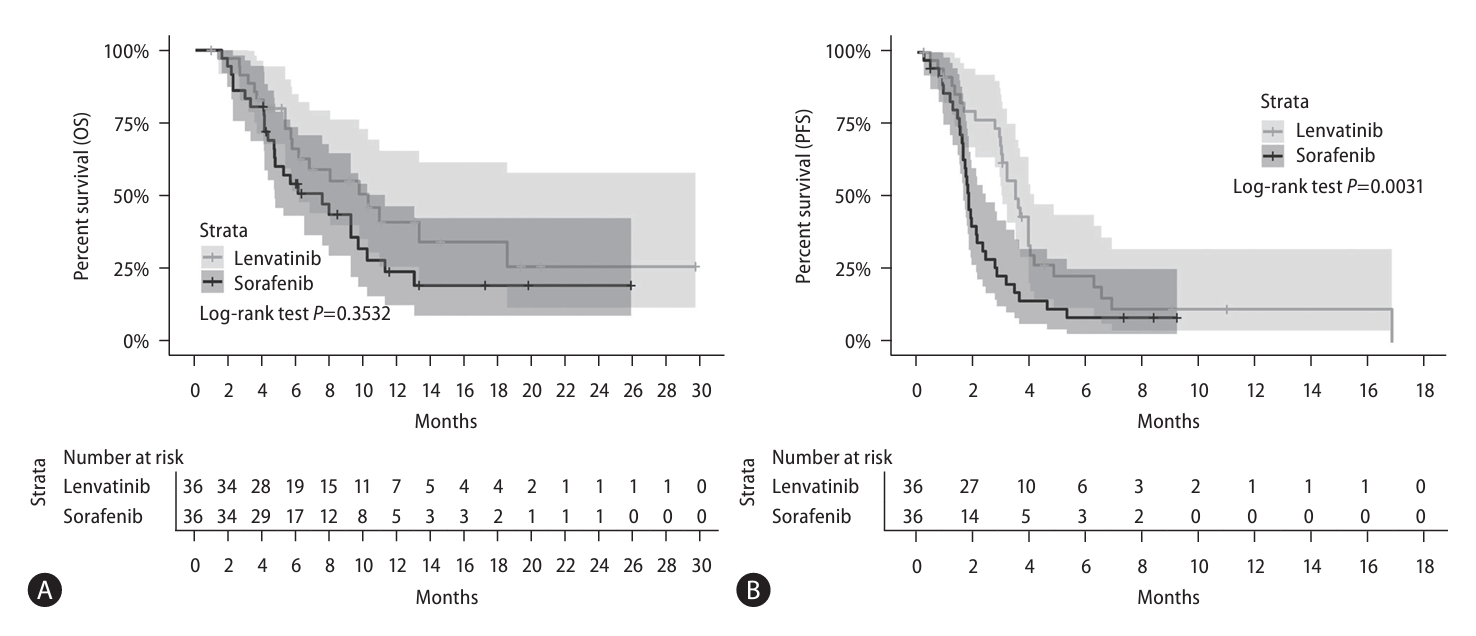
Table 1.
Values are presented as median (interquartile range), or number (%).
Matching variables: age, sex, tumor size, tumor number, extrahepatic metastasis or lymph node metastasis, Child-Pugh class.
ECOG PS, Eastern Cooperative Oncology Group performance status; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; PIVKA-II, prothrombin-induced by vitamin K absence or antagonist-II.
Table 2.
Table 3.
Table 4.
Table 5.
- Abbreviations
- Abbreviations
ATE+BEV atezolizumab plus bevacizumab
HCC hepatocellular carcinoma
PFS progression-free survival
OS overall survival
TKI tyrosine kinase inhibitor
HR hazard ratio
AFP alpha-fetoprotein
PIVKA-II protein induced by vitamin K antagonist-II
HBV hepatitis B virus
HCV hepatitis C virus
BCLC Barcelona Clinic Liver Cancer
RECIST 1.1 Response Evaluation Criteria in Solid Tumors 1.1
ORR objective response rate
CR complete response
PR partial response
DCR disease control rate
SD stable disease
AEs adverse events
IQR interquartile range
PS propensity score
HFS hand-foot syndrome
- REFERENCES
- REFERENCES
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-386.
[Article] [PubMed]2. Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol 2018;24:1-9.
[Article] [PubMed]3. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835-853.
[Article] [PubMed]4. Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302-309.
[Article] [PubMed]5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390.
[Article] [PubMed]6. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34.
[Article] [PubMed]7. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-1173.
[Article] [PubMed]8. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.
[Article] [PubMed]9. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:541-565.
[PubMed]10. Su GL, Altayar O, O’Shea R, Shah R, Estfan B, Wenzell C, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology 2022;162:920-934.
[Article] [PubMed]11. Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer 2022;3:386-401.
[Article] [PubMed] [PMC]12. Chen CT, Feng YH, Yen CJ, Chen SC, Lin YT, Lu LC, et al. Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment. Hepatol Int 2022;16:1199-1207.
[Article] [PubMed]13. Yoo C, Kim JH, Ryu MH, Park SR, Lee D, Kim KM, et al. Clinical outcomes with multikinase inhibitors after progression on firstline atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: A multinational multicenter retrospective study. Liver Cancer 2021;10:107-114.
[Article] [PubMed] [PMC]14. Chen YH, Chen YY, Wang JH, Hung CH. Efficacy and safety of lenvatinib after progression on first-line atezolizumab plus bevacizumab treatment in advanced hepatocellular carcinoma patients. Anticancer Res 2023;43:1377-1384.
[Article] [PubMed]15. Falette-Puisieux M, Nault JC, Bouattour M, Lequoy M, Amaddeo G, Decaens T, et al. Beyond atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: overall efficacy and safety of tyrosine kinase inhibitors in a real-world setting. Ther Adv Med Oncol 2023;15:17588359231189425.
[Article] [PubMed] [PMC]16. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
[Article] [PubMed]17. Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1 - Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur J Cancer 2016;62:138-145.
[Article] [PubMed] [PMC]18. Persano M, Rimini M, Tada T, Suda G, Shimose S, Kudo M, et al. Sequential therapies after atezolizumab plus bevacizumab or lenvatinib first-line treatments in hepatocellular carcinoma patients. Eur J Cancer 2023;189:112933.
[PubMed]19. Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: A real-world, multi-center study. Cancers(Basel) 2022;14:1747.
[Article] [PubMed] [PMC]20. Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, et al. Lenvatinib as second-line treatment after atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma: Clinical results show importance of hepatic reserve function. Oncology 2023;101:624-633.
[Article] [PubMed]21. Yano S, Kawaoka T, Yamasaki S, Johira Y, Kosaka M, Shirane Y, et al. Therapeutic efficacy and safety of lenvatinib after atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Cancers(Basel) 2023;15:5406.
[Article] [PubMed] [PMC]22. Palmer ME, Gile JJ, Storandt MH, Jin Z, Zemla TJ, Tran NH, et al. Outcomes of patients with advanced hepatocellular carcinoma receiving lenvatinib following immunotherapy: A real world evidence study. Cancers(Basel) 2023;15:4867.
[Article] [PubMed] [PMC]23. Cheon J, Ryoo BY, Kang B, Chon H, Yoo C. Phase II trial of second-line regorafenib in patients with unresectable hepatocellular carcinoma after progression on first-line atezolizumab plus bevacizumab: REGONEXT trial. J Clin Oncol 2023;41(4 Suppl):TPS634-TPS634.
[Article]24. Chan SL, Ryoo BY, Mo F, Cheon J, Li L, Wong KH, et al. A phase II clinical trial to study the use of cabozantinib (cabo) in patients with hepatocellular carcinoma (HCC) post immunotherapy treatment. J Clin Oncol 2023;41(4 Suppl):571-571.
[Article]25. Frenette C. How to choose second-line treatment for hepatocellular carcinoma. Clin Adv Hematol Oncol 2021;19:726-729.
[PubMed]