INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic liver disease. Interferon therapy for chronic hepatitis C has a relatively low rate of sustained viral response (SVR) of only 10-20% after 48 weeks [1]. Pegylated interferon (PegIFN) combined with ribavirin was the main therapy for chronic hepatitis C with an overall SVR rate of about 40-60% for genotype 1 and 70-80% for genotypes 2 and 3 [2,3]. However, the combination of standard PegIFN-╬▒ and ribavirin has strict indications and various adverse effects. In clinical situations, only 20-30% of patients with chronic hepatitis C are treated with PegIFN-╬▒ and ribavirin [4]. Moreover, less than 5% of the HCV-infected population worldwide is aware of their infection, and only about 3% are treated with antiviral therapy [5].
Recently developed directly acting antivirals (DAAs) therapies have shown remarkable SVR rates above 90% with minimal adverse events. HCV eradication is suggested as a realistic goal of treatment by many hepatologists [6], but it remains a substantial challenge [5] because acute and chronic hepatitis C virus infection are largely asymptomatic and comprehensive screening programs are rare in highly endemic regions. Controlling HCV requires a combination of identifying infections, proper treatment, and effective prevention. Despite highly efficient oral DAA therapy, the clinical eradication of HCV has been suggested to be difficult due to various unexpected barriers to treatment. Therefore, the aim of our study was to investigate the proportion of patients with and reasons for PegIFN-╬▒ and ribavirin ineligibility or failure, and to determine if these factors would also potentially become a barrier to treatment with DAAs.
MATERIALS AND METHODS
Study population
The medical records of 1,277 patients who were tested for anti-HCV antibodies or HCV ribonucleic acid (RNA) levels at Kosin University Gospel Hospital in Busan, Korea from January 2009 to December 2013 were reviewed. All patients were evaluated by one of three hepatologists for diagnosis and treatment planning. Decisions about treatment were made according to the Korean Association for the Study of the Liver clinical practice guidelines.
The study exclusion criteria were decompensated cirrhosis, poorly controlled psychiatric disorder, extra-hepatic transplantation, autoimmune disease, uncontrolled thyroid disease, symptomatic cardiopulmonary disease, uncontrolled diabetes, uncontrolled anemia (hemoglobin <10 g/dL), neutropenia (absolute neutrophil count <750/mm3), thrombocytopenia (platelet <50,000/mm3), active alcohol or drug use, cancer or unwillingness to undergo treatment.
Study design
The variables collected were treatment initiation, treatment results and reasons for stopping or not receiving treatment. Patients were classified as noncompliant if they had no follow-up visit or missed visits frequently not enough to receive proper treatment. Statistical analysis was conducted using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA) and P-value <0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
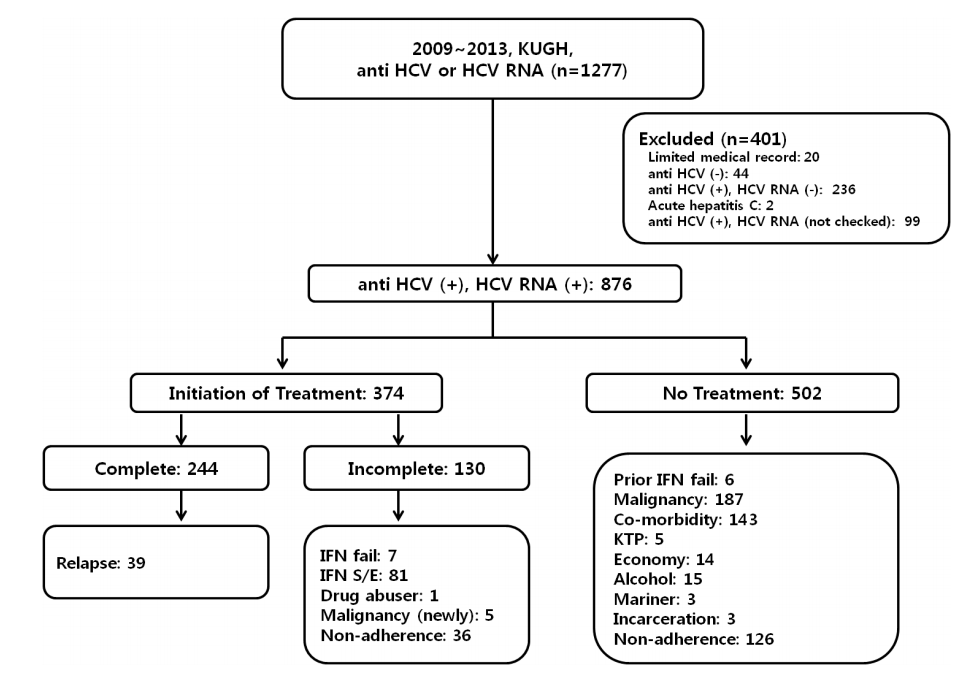
Between 2009 and 2013, anti-HCV antibody or HCV RNA laboratory tests were performed for 1,277 patients at Kosin University Gospel Hospital. Of these, 401 patients were excluded from this study. Two patients were diagnosed with acute hepatitis C, 44 were negative for anti-HCV antibody, 236 were positive for antiHCV antibody but negative for HCV RNA, and 20 had limited medical records (Fig. 1). Despite being positive for anti-HCV antibody, 99 patients did not have HCV RNA checked because of diagnosed malignancy (n=57), severe medical or psychiatric illness (n=21), financial status (n=1), ongoing alcohol abuse (n=2), and not following medical advice (n=18).
A total of 876 patients were confirmed positive for both anti-HCV antibody and HCV RNA and diagnosed with chronic hepatitis C. Three-hundred seventy-four patients (42.6%) started interferon-based antiviral therapy. The patients who were treated with interferon therapy were younger than the non-treated group (P<0.001). Genotype 2 was more prevalent in the treated group (P=0.009) (Table 1). Two-hundred forty-four (28%) patients completed treatment with combined standard PegIFN-╬▒ and ribavirin, and 39 (16%) patients were diagnosed with HCV relapse. Overall treatment response irrespective of genotype was 72.9%. Virological responses according to genotype were similar to previous reports [3]. SVR for genotype 1 was 66.9 %, genotype 2 was 77.8% (Table 2).
Reasons for stopping or preventing interferon-based therapy
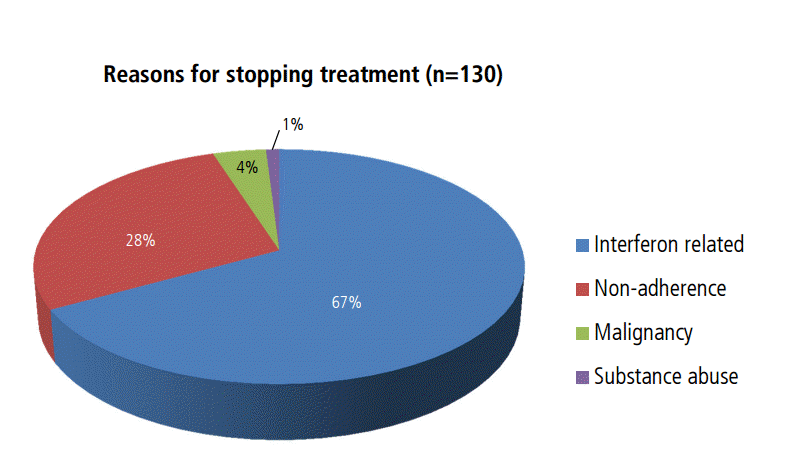
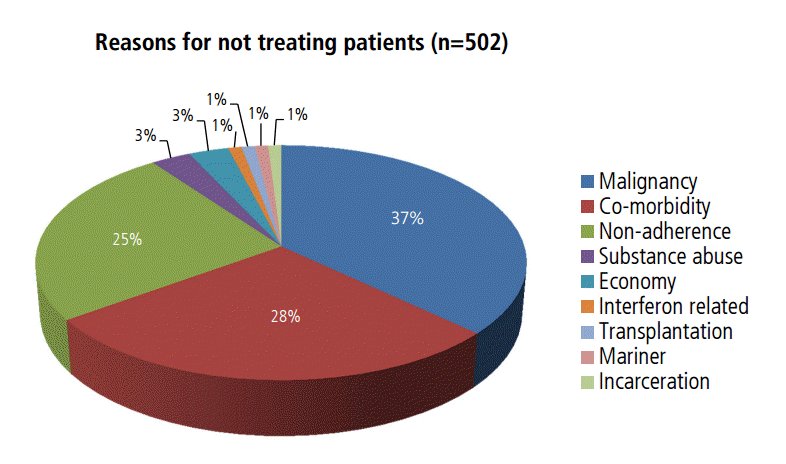
One hundred thirty patients did not complete 3 or 6 months of treatment for various reasons: adverse effects of interferon-based therapy (n=81, 62%), poor response to interferon-based therapy (n=7, 5%), noncompliance with medical advice or loss to follow-up (n=36, 27%), newly developed malignancy (n=5, 4%), or active alcohol abuse (n=1, 0.7%) (Fig. 2). A total of 502 patients could not initiate interferon-based antiviral therapy because of malignancy (n=187, 37%), medical contraindications (n=143, 28%), not following medical recommendations (n=126, 25%), financial status (n=14, 3%), ongoing alcohol use (n=15, 3%), failing prior interferon-based therapy (n=6, 1%), kidney transplantation (n=5, 1%), mariner (n=3), and incarceration (n=3) (Fig. 3).
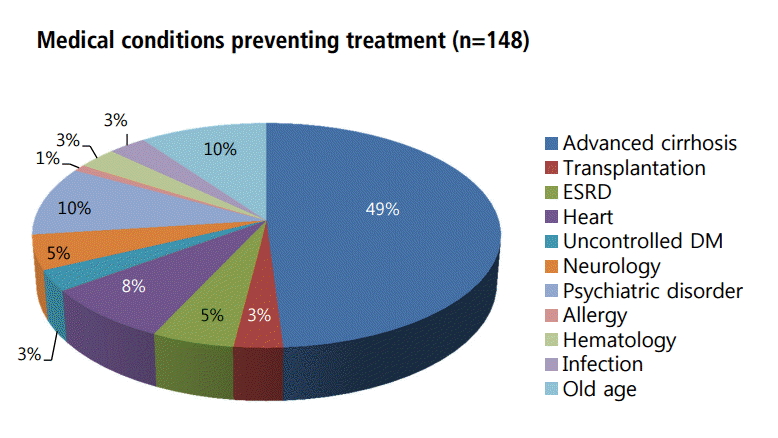
Malignancy was the most common reason for stopping or not receiving interferon-based therapy for chronic hepatitis C in our study. Of the 192 patients with malignancy, 156 (81%) had hepatocellular carcinoma (HCC), 15 (8%) had gastrointestinal cancer, and 6 (3%) had pancreaticobiliary cancer (Table 3). Of the 148 patients who did not receive interferon-based antiviral therapy due to medical conditions, 49% had advanced liver cirrhosis, 10% were elderly, 10% had a poorly controlled psychiatric disorder, 8% had symptomatic cardiac disease, 5% had end-stage renal disease, 3% had kidney transplantation, 3% had uncontrolled diabetes, 3% had hematologic disease, and 3% had severe infection (Fig. 4).
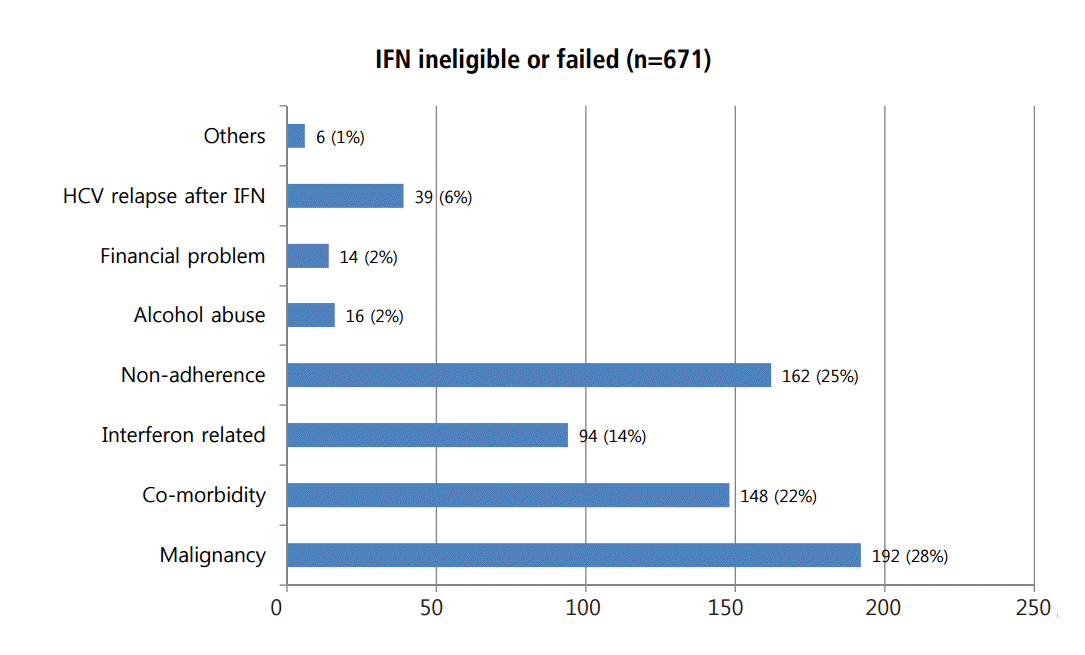
Of the 192 patients who did not initiate or complete interferon-based antiviral therapy for nonmedical reasons, 162 (26%) were noncompliant with evaluation, education, and visit appointments; 16 (3%) were actively abusing alcohol, and 14 (2%) had financial difficulties. Other complicating reasons included mariner or incarceration (Fig. 5).
DISCUSSION
The prevalence of blood-borne HCV infection is estimated to be about 3% worldwide. Although DAAs that target nonstructural protein of HCV have potent antiviral efficacy and few adverse effects, the eradication of HCV is supposed to be difficult because most acute and chronic HCV infection is asymptomatic, and screening programs are limited in most countries. In a prospective, multicenter cohort at five university hospitals from January 2007 to December 2011, 1,173 patients age >18 years who were positive for anti-HCV antibody were enrolled to investigate the epidemiological and clinical characteristics of HCV infection of Korea. The rate of antiviral therapy for HCV was 42.8% of the HCV cohort [7]. At our center, 876 patients were diagnosed in a chronic viremic state, and 374 (42.6%) were treated with interferon-based therapy.
PegIFN-╬▒ and ribavirin combination therapy have absolute contraindications including uncontrolled depression; psychosis or epilepsy; pregnancy or couples unwilling to use adequate contraception; severe concurrent medical diseases; and co-morbidities including retinal disease, autoimmune thyroid disease, and decompensated liver disease [8]. After considering the contraindications for interferon-based therapy, a large number of patients diagnosed with chronic hepatitis C could not have treatment. Therefore, the proportion of patients initiating treatment was 31.0% in Swiss cohort study [9], and 33.0% in Danish cohort study [10].
According to Falck-Ytter et al. [4], 72% of chronic hepatitis C patients were not treated. Of these, 37% did not adhere to medical recommendations, 34% were medically or psychologically ineligible, 13% had ongoing alcohol or drug abuse, and 11% refused treatment. Only 28% of chronic hepatitis C patients were treated and 13% had a sustained viral response. Narasimhan et al. [11] retrospectively reviewed the charts of all HCV patients who underwent liver biopsies. About 60% were not treated with interferon-based therapy because of loss to follow-up or non-compliance (31%), patient preference (22%), etc. In other words, chronic hepatitis C treatment largely depended on patientsŌĆÖ intention to treat, not medical decisions made by doctors. Restrepo et al. [12] reported that approximately 85% of patients co-infected with HCV and human immunodeficiency virus were not treated. Of these, 40% were noncompliant with medical schedules, 15% were actively abusing drugs or alcohol, and 15% refused antiviral treatment.
In our study, 876 patients were diagnosed with chronic hepatitis C having positive results for both anti-HCV and HCV RNA. Of the 876 patients, 244 were eligible for interferon, but 39 (16%) diagnosed with HCV relapse. 632 patients could not be treated appropriately with interferon-based antiviral therapy (Fig. 1). The reasons for stopping or not receiving interferon-based antiviral treatment include malignancy (30%), co-morbidity (23%), interferon-related reasons (15%) and non-adherence (26%).
The most prevalent malignancy in our cohort was HCC (81%) (Table 2). In the interferon era, HCC was a relative contraindication for antiviral therapy; however, treating chronic hepatitis C with interferon was considered to reduce HCC recurrence and improve survival [13]. The recent introduction of DAA has been expected to reduce the incidence of HCC in HCV-related cirrhotic livers. Unfortunately, DAA-induced clearance of HCV was not able to reduce the occurrence of HCC in patients with HCV-related cirrhotic livers [14]. In spite of DAA treatment, high rate of early HCC recurrence was noted in patients previously treated for HCC [14,15]. Therefore DAA therapy in HCC patients has yet to be determined.
Patients with severe co-morbidities could not be treated with interferon-based therapy because of possible severe adverse effects. About 49% of patients had advanced or decompensated liver cirrhosis that was a contraindication to interferon therapy (Fig. 4). However, an oral DAA regimen could be safe and highly effective in treating patients who are ineligible for interferon-based antiviral therapy due to HCV-related cirrhosis, decompensation. In addition to advanced liver diseases, DAA could be administered safely in patients with symptomatic cardiovascular disease, chronic renal disease, uncontrolled diabetes, extra-hepatic transplantation and psychiatric disorders [16].
Non-adherence, financial problems and alcohol abuse are also reasons to prevent antiviral therapy. With short treatment duration and all oral regimens, improved compliance is expected in patients using DAA. On the other hand, DAA is so expensive that it would be difficult for a physician to prescribe to all chronic hepatitis C patients. Therefore, a part of compliance and socioeconomic problems would be remained in DAA era.
Therefore, we could suppose that at least 42% of patients who were ineligible or failed to interferon-based antiviral therapy due to co-morbidity (22%), interferon intolerability (14%) and HCV relapse (6%) would be treated with DAAs. However, a large portion of patients who were non-adherent (25%), had alcohol abuse (2%), or had financial concerns (2%) would not be able to get a chance to use the DAA (Fig. 5). Unlike hepatitis B virus-related HCC, patients with HCC occurred with HCV (23%, 156/671) have not been determined to be treated with DAA. This study has some limitations, including a retrospective design and that it was conducted in a single, tertiary hospital. The combined co-morbidity and malignancy rates were higher than in other Korean studies [7,17]. Therefore, the results cannot be accepted generally. In spite of the limitation, this study has a clear message considering limitation of DAA therapy in chronic hepatitis C patients who were ineligible or failed to interferon based antiviral therapy.
In conclusion, only 27.7% of patients diagnosed with chronic hepatitis C in our study were treated with an interferon-based regimen. With the advent of DAAs, at least 42% of patients who were ineligible or failed to interferon and experienced HCV relapse would be able to use DAA. However nonmedical reasons, including noncompliance, financial problems, substance abuse and hepatocelluar carcinoma remain obstacles to treat chronic hepatitis C. Our study suggests that, in spite of DAA development, eradicating HCV would be difficult due to various unexpected reasons.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




