| Clin Mol Hepatol > Volume 19(2); 2013 > Article |
Hepatocellular adenoma (HCA) is a benign liver tumor composed of hepatocytes.1 HCA represents a heterogeneous entity, and is subclassified into four groups according to genotype and phenotype: hepatocyte nuclear factor (HNF) 1╬▒-inactivated HCA, ╬▓-catenin-activated HCA, inflammatory HCA, and unclassified HCA.1 Theses subtypes vary greatly in their clinical and pathological features. Identifying the HCA subtype is important because it represents a way to understand the prognosis of HCA in terms of hepatocellular carcinoma (HCC) transformation. We present a case of ╬▓-catenin-activated HCA and discuss the histopathological findings with a review of the literature.
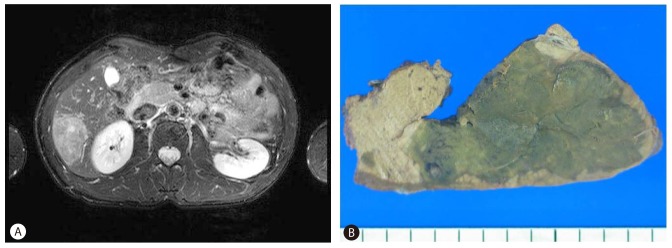
A 39-year-old man was admitted to our hospital to evaluate an asymptomatic hepatic mass detected incidentally during a health checkup. The patient did not have a history of alcoholism, androgenic steroid use, or metabolic syndrome. The subject's body mass index (BMI, kg/m2) was 20.7 (normal range, 18.50-24.99 kg/m2). Serum aspartate aminotransferase was 21 IU/L (normal range, 12-33 IU/L), alanine aminotransferase was 14 IU/L (normal range, 5-35 IU/L), and gamma-glutamyl transferase (GGT) was 31 IU/L (normal range, 12-73 IU/L). Other liver function tests were within normal limits. Hepatitis B virus surface antigen was negative, and the levels of tumor markers were all within normal ranges. Magnetic resonance imaging demonstrated a lobulated contoured hepatic mass located in segment VI that showed iso-intensity on T1-weighted images, heterogeneous high-intensity on T2-weighted images, and a focal signal drop, particularly at the periphery, on fat-suppressed T2-weighted images (Fig. 1A), suggesting hepatocellular adenoma. A laparoscopic wedge resection was performed.
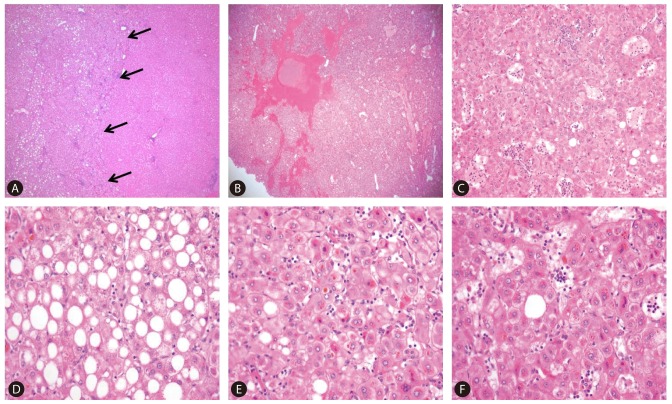
A 5.2├Ś3.6 cm sized, homogeneous, green colored hepatic mass was observed on the cut section (Fig. 1B). The margins of the mass were relatively well-defined but not encapsulated. The mass was microscopically composed of benign hepatocytes arranged in one or two thick irregular cell plates with sharp demarcation from the surrounding non-tumoral liver (Fig. 2A). The background liver was normal. Other characteristic pathological features of this mass included peliosis (Fig. 2B), sinusoidal dilatation and inflammatory infiltrates (Fig. 2C), diffuse steatosis (Fig. 2D), cytoplasmic bile pigment and feathery degeneration of hepatocytes (Fig. 2E), numerous unpaired arteries, and bile duct loss. An occasional pseudoglandular growth pattern and cytologic atypia were also observed (Fig. 2F). Immunohistochemistry revealed that the tumor cells were positive for glutamine synthetase (GS) in a diffuse pattern (Fig. 3A) and showed aberrant cytoplasmic and nuclear ╬▓-catenin expression (Fig. 3B). The tumor cells were negative for two inflammation-related proteins, serum amyloid A and C reactive protein (SAA/CRP) and also negative for glypican 3. The final diagnosis was ╬▓-catenin-activated HCA based on the histological and immunohistochemical findings. No obvious recurrence of the tumor was detected post-operatively during the 7 month follow-up.
HCA is a rare benign liver tumor (solitary or multiple) that most frequently develops in young women who are using oral contraceptives (OC). HCA is rare in children, men, and the elderly.1 Most men developing HCA have been users of anabolic steroids for bodybuilding.1,2 Patients taking androgens for Fanconi anemia or acquired aplastic anemia are also at risk.2 Obesity and alcohol intake have recently been shown to be risk factors for developing inflammatory HCA.3 HCA can also occur in specific settings such as metabolic disease (glycogenosis, tyrosinemia, or galactosemia) but rarely in association with polycystic ovary syndrome or advanced-stage fatty liver disease.4 Some small HCA may regress after withdrawal of OC or after menopause; however, they usually remain stable, or increase in size. Clinically significant hemorrhaging is observed in 20-25% of cases. Malignant transformation to HCC is rare but well-documented.5-7
HCA are classified into three main subgroups according to genotype: (1) inactivating mutations in the HNF 1╬▒ gene, (2) activating mutation in the CTNNB1 gene that encodes ╬▓-catenin, and (3) activation of signal transducers and activators of transcription type 3 in the absence of ligand due to glycoprotein 130 mutants.3-5,7 A phenotypic classification is based on features of morphology and immunohistochemistry. Close relationships have been found between these molecular subgroups defined by genotype and their clinicopathological characteristics.3-5,7 This new genotype/phenotype subclassification of HCA was introduced by Bioulac-Sage and colleagues and is currently presented by the WHO Tumors of the Digestive System.1,5,7 The HNF 1╬▒ biallelic somatic mutation is observed in 35-40% of HCA cases. It almost always occurs in women. HNF 1╬▒-inactivated HCA is most highly steatotic, with a lack of expression of liver fatty acid binding protein (LFABP) in immunohistochemistry analyses. Significant inflammation and cellular atypia are absent. A HNF 1╬▒ germline mutation is observed in <5% of HCA cases and can be associated with MODY 3 diabetes, particularly in children and young adults. The diagnosis of HNF 1╬▒-inactivated HCA may be suggested by a radiologist due to the steatosis. The second type, an activating ╬▓-catenin mutation, is found in 10-15% HCA cases. This ╬▓-catenin activated HCA subtype is often associated with males as in our case or specific conditions (glycogenosis or administration of male hormone). Cytologic atypia and a pseudoglandular growth pattern are frequently observed. Immunohistochemistry studies show that this HCA subtype overexpresses ╬▓-catenin (nuclear and cytoplasmic) and GS. Because this group has a higher risk of malignant transformation into HCC than that of other subtypes, identifying ╬▓-catenin activated HCA subtype is extremely important. The third, inflammatory HCA, is observed in 45-60% of cases, and most patients with this subtype are women. Lesions are characterized by inflammatory infiltrates, thick-walled arteries, sinusoidal dilatation, and a ductular reaction. Inflammatory HCA is immunohistochemically characterized by increased expression of SAA/CRP at both the mRNA and protein levels. GS is usually absent, and LFABP is normally expressed in the non-tumoral liver parenchyma. GGT is frequently elevated in this group, with a biological inflammatory syndrome present. Additionally, more overweight patients (BMI >25) are found in this group. A risk for malignant transformation is present, particularly for cases in which ╬▓-catenin is also mutated. Finally, <10% of HCA is unclassified with non-mutated HNF 1╬▒ or ╬▓-catenin and without an inflammatory infiltrate.
van Aalten et al reported that the subtype classification was straightforward in >90% of HCA cases using the immunohistochemical staining pattern and morphology.8 Our case showed diffuse and strong positivity for GS and aberrant cytoplasmic and nuclear staining for ╬▓-catenin, so the diagnosis of ╬▓-catenin-activated HCA was made by immunohistochemistry (Table 1). However, the present case exhibited some unusual histological features such as diffuse steatosis, inflammatory infiltrates, and peliosis, which usually appear in inflammatory HCA. Therefore, our case should be distinguished from ╬▓-catenin-activated inflammatory HCA. Steatosis and inflammation are usually absent in ╬▓-catenin-activated HCA.1,5,7,9 However, Bordeaux's group has pointed out that ╬▓-catenin-activated HCA and inflammatory HCA have overlapping features of an inflammatory infiltrate, patchy steatosis, diffuse GS staining, and ╬▓-catenin mutation.3 Inflammatory HCA is characterized by diffuse, strong expression of SAA or CRP in tumoral hepatocytes.1,3-5,7 These markers are useful for distinguishing cases with overlapping histological features.10 We excluded inflammatory HCA because no SAA/CRP expression was observed, which is necessary for a diagnosis of inflammatory HCA.
Despite that radiological imaging techniques have made great progress in identifying benign liver tumors, it is difficult to distinguish between different types of HCA (particularly ╬▓-catenin-activated HCA, which has variable and nonspecific radiologic findings).11 Complete surgical removal and a careful pathological examination are necessary to prevent a potential misdiagnosis and identify ╬▓-catenin-activated HCA, which is prone to develop into HCC.
REFERENCES
1. Bioulac-Sage P, Balabaud C, Wanless I. Focal nodular hyperplasia and hepatocellular adenoma. In: Bosman F, Carneiro F, Hruban R, Theise ND, eds. WHO Classification of Tumours of the Digestive System. 4th Ed. Lyon, France: IARC Press; 2010. p. 198-204.
2. Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol 2004;77:257-267. 15495253.


3. Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007;46:740-748. 17663417.


4. Bioulac-Sage P, Cubel G, Balabaud C. Pathological diagnosis of hepatocellular adenoma in clinical practice. Diagn Histopathol (Oxf) 2011;17:521-529.

5. Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 2009;50:481-489. 19585623.


6. Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod Pathol 2008;21:491-497. 18246041.



7. Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006;43:515-524. 16496320.


8. van Aalten SM, Verheij J, Terkivatan T, Dwarkasing RS, de Man RA, Ijzermans JN. Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J Hepatol 2011;55:120-125. 21145863.


9. Van der Borght S, Libbrecht L, Katoonizadeh A, Aerts R, Nevens F, Verslype C, et al. Nuclear beta-catenin staining and absence of steatosis are indicators of hepatocellular adenomas with an increased risk of malignancy. Histopathology 2007;51:855-856. 17903198.


Figure┬Ā1
(A) Focal areas of signal loss at the periphery on fat-suppressed T2-weighted images. (B) Cut section of the gross specimen showed well-demarcated, greenish mass-like lesion without hemorrhage or cystic change.
Figure┬Ā2
(A) The margin of the mass was non-encapsulated, and had a sharp demarcation (arrows) from the surrounding non-tumoral liver (hematoxylin and eosin [H & E] stain, ├Ś40). The histological examination showed (B) peliosis (H & E stain, ├Ś100), (C) sinusoidal dilatation and inflammatory infiltrates (H & E stain, ├Ś200), and (D) steatotic hepatocytes (H & E stain, ├Ś400). (E) Cytoplasmic bile pigment, feathery degeneration (H & E stain, ├Ś400), (F) an occasional pseudoglandular growth pattern and cytologic atypia (H & E stain, ├Ś400) were also observed.
Figure┬Ā3
(A) Glutamine synthetase was strongly and diffusely expressed by tumoral hepatocytes (├Ś200), and (B) Aberrant cytoplasmic and nuclear expression of ╬▓-catenin by tumoral hepatocytes (arrows) (├Ś400).

Table┬Ā1.
| HNF 1╬▒-inactivated HCA (35-40%) | ╬▓-catenin activated HCA (10-15%) | Inflammatory HCA* (45-60%) | Unclassified HCA (10%) | Present case | |
|---|---|---|---|---|---|
| Clinical features | |||||
| Genetic background | HNF 1╬▒ inactivation Somatic (90%) | ╬▓-catenin mutation | Glycoprotein 130 mutation | Unidentified | ND |
| Germline (10%) | |||||
| Clinical relevance | Mainly women, MODY 3 diabetes | Male, anabolic steroids, gycogenosis high risk of HCC | Male can be involved, obesity, alcohol, risk of HCC | Male, normal BMI, non-alcoholism | |
| Histological features | |||||
| Steatosis | Usually 3+ | - | - to 2+ (heterogeneous) | + / - | 2+ |
| Ductular reaction | - | - | +/- to 3+ | - | - |
| Sinusoidaldilatation | - to + | - to +/- | - to 3+ | - | 2+ |
| Abnormal thick arteries | - | - | + to 2+ | - to +/- | - |
| Inflammatory infiltrates | - to +/- | - | + to 3+ | -/+ | 2+ |
| Cytologic atypia | - | + | -ŌĆĀ | - | + |
| Peliosis | - to +/- | - to +/- | - to 2+ | - to +/- | + |
| Immunohistochemistry | |||||
| Glutamine synthetase | - | + | -ŌĆĀ | - | + |
| ╬▓-cateninŌĆĪ | - | + | -ŌĆĀ | - | + |
| SAA/CRP | - | - | + to 3+ | - | - |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




