| Clin Mol Hepatol > Volume 29(4); 2023 > Article |
|
See the commentary-article "Letter regarding ŌĆ£Evidence-based hyponatremia management in liver diseaseŌĆØ" on page 1043.
ABSTRACT
Hyponatremia is primarily a water balance disorder associated with high morbidity and mortality. The pathophysiological mechanisms behind hyponatremia are multifactorial, and diagnosing and treating this disorder remains challenging. In this review, the classification, pathogenesis, and step-by-step management approaches for hyponatremia in patients with liver disease are described based on recent evidence. We summarize the five sequential steps of the traditional diagnostic approach: 1) confirm true hypotonic hyponatremia, 2) assess the severity of hyponatremia symptoms, 3) measure urine osmolality, 4) classify hyponatremia based on the urine sodium concentration and extracellular fluid status, and 5) rule out any coexisting endocrine disorder and renal failure. Distinct treatment strategies for hyponatremia in liver disease should be applied according to the symptoms, duration, and etiology of disease. Symptomatic hyponatremia requires immediate correction with 3% saline. Asymptomatic chronic hyponatremia in liver disease is prevalent and treatment plans should be individualized based on diagnosis. Treatment options for correcting hyponatremia in advanced liver disease may include water restriction; hypokalemia correction; and administration of vasopressin antagonists, albumin, and 3% saline. Safety concerns for patients with liver disease include a higher risk of osmotic demyelination syndrome.
Hyponatremia is the most common electrolyte disturbance encountered in clinical practice and is associated with increased mortality and morbidity rates [1]. Liver disease, especially cirrhosis, is a relatively frequent etiology of hyponatremia. Hyponatremia in liver cirrhosis is typically defined as a serum sodium (sNa) concentration <135 mmol/L; however, some experts have defined hyponatremia as an sNa concentration <130 mmol/L [2,3]. The prevalence of sNa concentrations <135 mmol/L, <130 mmol/L, <125 mmol/L, and <120 mmol/L are 49.4%, 21.6%, 5.74%, and 1.2% in patients with liver cirrhosis, respectively [3]. Several studies have confirmed that hyponatremia in advanced liver cirrhosis is associated with poor outcomes, including refractory ascites (RA), hepatic encephalopathy (HE), hepatorenal syndrome (HRS), and mortality in advanced liver disease [4-6]. Given that sNa levels have been integrated into the model for the end-stage liver disease (MELD) score, which is used to determine liver transplantation (LT) priority, hyponatremia management is crucial [7].
Herein, we review the causes, factors, clinical features, and pathophysiology of hyponatremia in patients with liver disease. We also discuss the general treatment for hyponatremia in this patient population and management of hyponatremia before LT.
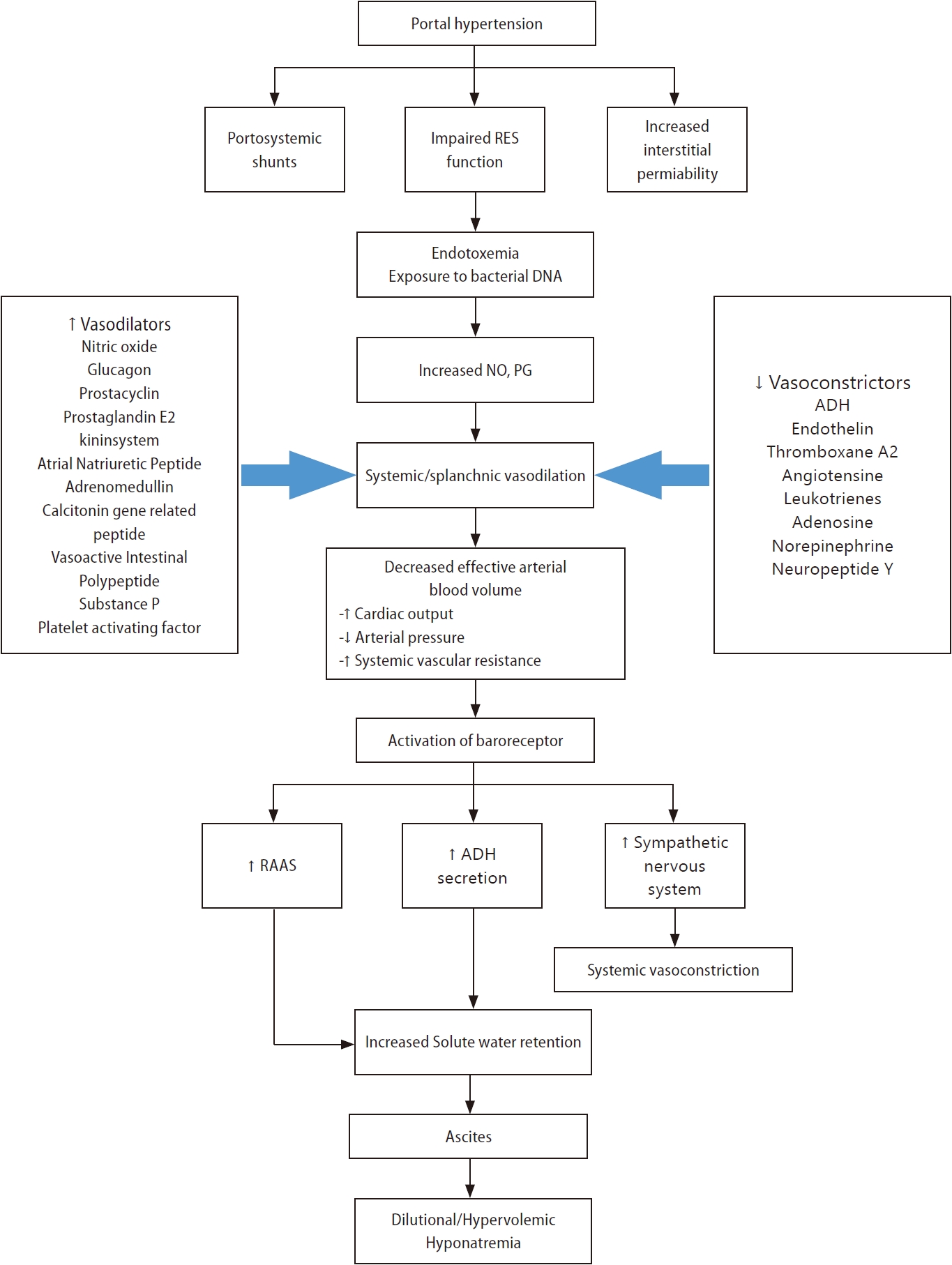
The mechanisms of hyponatremia are mainly cirrhosis-induced hemodynamic compromise and other pathogenetic or superimposed factors.
The primary pathophysiologic mechanisms that lead to hypervolemic hyponatremia in patients with cirrhosis are systemic vasodilation and arterial underfilling [8]. Hyperdynamic circulation, resulting in increased cardiac output, which markedly reduces systemic vascular resistance and decreases mean arterial pressure, is common among patients with cirrhosis and progressive portal hypertension [9,10]. A significant decrease in vascular resistance is mainly associated with splanchnic vasodilation, owing to an increase in vasodilators and an opening of portosystemic collaterals [8,11]. Among vasodilators, the most important mediator is nitric oxide released by endothelial cells. Other vasodilators, such as glucagon, vasoactive intestinal peptide, substance P, platelet-activating factor, prostaglandins, prostacyclin, hydrogen sulfide, endocannabinoids, adrenomedullin, and endothelium-derived hyperpolarizing factors are involved in splanchnic vasodilation [12,13]. Vascular endothelial growth factors, tumor necrosis factor-alpha, mechanical stimuli due to shear stress, and decreased endotoxin or bacterial DNA clearance exist in the gastrointestinal tract due to dysfunction of the reticuloendothelial function and portal-systemic shunting [12,14,15]. Extrahepatic hyporeactivity to vasoconstrictors (endothelin-1, thromboxane A2, leukotrienes, norepinephrine, and antidiuretic hormone [ADH]) also contributes to systemic vasodilation, which is mediated by G protein-coupled transmembrane receptors [14].
Systemic or splanchnic vasodilation and lack of arterial filling cause decreased carotid and renal baroreceptor pressure in patients with cirrhosis and portal hypertension [14]. As pressure is reduced due to a decrease in the effective circulation volume, sodium retention mechanisms, such as the renin-angiotensin-aldosterone system, sympathetic nervous system, and ADH, are activated to retain sodium and water [16-19]. Although these factors increase extracellular sodium stores, plasma volume, and cardiac output, the net effect is sodium and water reabsorption by the kidneys because the patient is substantially depleted of body fluid [15,20]. Serum osmolality is generally low in patients with decompensated cirrhosis; thus, ADH must be suppressed by osmotic stimulation [15]. However, non-osmotic stimuli act predominantly over osmotic stimuli to cause hyponatremia in patients with decompensated liver cirrhosis [15]. ADH released in response to hypovolemia in liver cirrhosis increases water permeability in the collecting ducts of the kidneys via aquaporin 2, a water channel protein [21-26]. This is one of the mechanisms leading to hypervolemic, dilutional hyponatremia in liver cirrhosis [14,15].
Kidney failure related to end-stage liver disease or acute kidney injury, including HRS, impairs urine dilution and causes dilutional hyponatremia accompanied by water retention [1,27-29]. Nephrotic syndrome due to hepatitis B or C viral infection causes hyponatremia [27,30,31]. Coexistent cardiac disorders, including heart failure (HF) or cardiomyopathy (e.g., alcoholic or hemochromatosis) in patients with liver disease, may result in hyponatremia via neurohormonal mechanisms [27,32].
Depletion of effective arterial volume via non-renal sodium loss (lactulose-associated diarrhea or vomiting), renal sodium loss (diuretics such as spironolactone/eplerenone), or primary adrenal insufficiency (autoimmune adrenalitis in autoimmune hepatitis or adrenal tuberculosis in alcoholics) can markedly increase secondary ADH release leading to water retention [1,27,33].
Syndrome of inappropriate antidiuresis (SIAD) may be caused by infection or medications used in the treatment of liver disease (terlipressin [34-36], interferon, brivanib [37], sorafenib [37], cixutumumab [38], and boceprevir [39]), acute intermittent porphyria, and malignancy (hepatocellular carcinoma) [27]. Endocrine disorders, including secondary adrenal insufficiency (caused by the pituitary gland or hypothalamus) or severe hypothyroidism, are rare causes of hyponatremia in patients with liver disease.
The accurate classification of hyponatremia in patients with liver disease is crucial for appropriate diagnosis and management. Hyponatremia is commonly classified based on the sNa concentration, severity of clinical symptoms, duration, and extracellular fluid (ECF) status (Table 1).
According to the severity of clinical symptoms, hyponatremia can be divided into either moderate (nausea, confusion, and headache) or severe/profound (vomiting, seizure, cardiopulmonary collapse, and coma). Based on the duration, hyponatremia can be differentiated as acute (<48 h) or chronic (>48 h) [1]. Patients with acute hyponatremia develop neurological symptoms due to cerebral edema because they do not have enough time to develop mechanisms that can prevent cerebral edema [40]. However, most patients with advanced cirrhosis have chronic hyponatremia, which allows for astrocytes to adapt and thus symptoms may not appear, making a diagnosis based on symptoms difficult [15].
Hyponatremia is classified into three types according to their ECF status: hypervolemic, euvolemic, and hypovolemic hyponatremia [41-43]. Patients with liver cirrhosis commonly have hypervolemia (increased ECF volume), characterized by ascites, anasarca, and/or pedal edema [2,15,20]. Hypervolemia is caused by sodium retention with impaired free-water excretion, resulting from renin-angiotensin-aldosterone or sympathetic nervous system activity and ADH hypersecretion, leading to dilutional hyponatremia [2,15]. Euvolemic hyponatremia is rare except when with SIAD or endocrine disorders, including adrenal insufficiency and severe hypothyroidism. Some patients (<10%) with liver cirrhosis have hypovolemic hyponatremia due to ECF loss after diuretic use or diarrhea/vomiting, characterized by a lack of ascites and pedal edema [44].
Based on a comprehensive assessment of the history (recent body fluid loss, high water intake with low salt, presence of a malignant tumor, recent surgery, and use of drugs [thiazide diuretics, intravenous immune globulin, and terlipressin], physical examination (detailed neurologic evaluation and assessment of ECF volume), and laboratory findings (serum and urine osmolality and urine sodium), the physician can determine the underlying cause of hyponatremia and adjust the treatment accordingly for patients with liver disease [45-47].
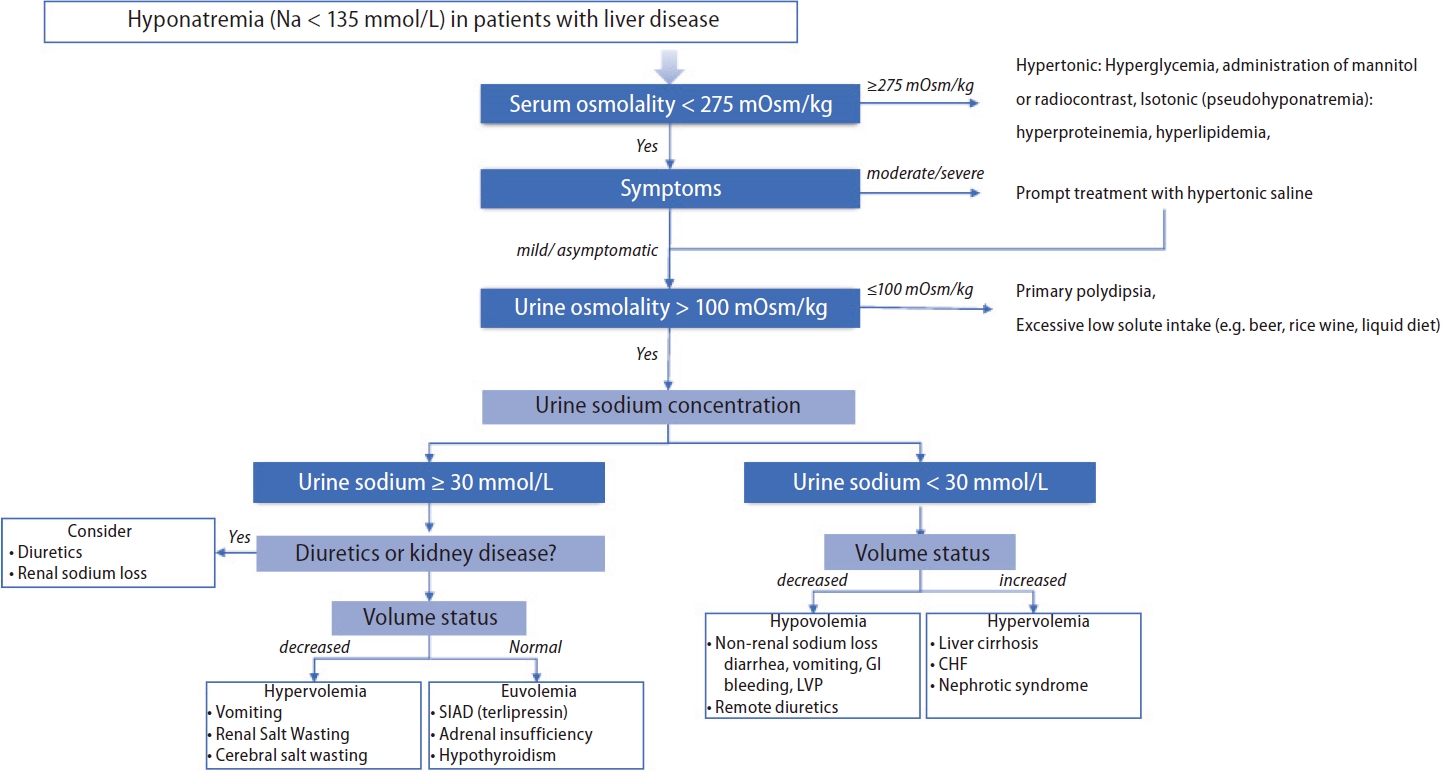
A practical diagnostic approach to hyponatremia, which is similar to that used for the general population, is utilized for patients with liver disease. The step-by-step process is presented below (Fig. 2).
The main objective of this step is to determine whether the hyponatremia is hypotonic. The serum osmolality is measured to differentiate hypotonicity [1]. For serum osmolality that is <275 mOsm/kg (hypotonic hyponatremia), further predefined steps are followed, while for serum osmolality that is Ōēź275 mOsm/kg, hyponatremia may be hypertonic (usually from hyperglycemia) or isotonic (due to pseudohyponatremia). In patients with hyperglycemia, the corrected sNa concentrations are calculated based on equations (1) and (2) [48,49]:
Pseudohyponatremia is defined as a spurious decrease in plasma sodium (<135 mmol/L) due to marked hyperproteinemia or hyperlipidemia [1]. Hyperproteinemia with increased globulin due to autoimmune hepatitis (elevated immunoglobulin G), primary biliary cirrhosis, alcoholic liver disease (elevated immunoglobulin M and immunoglobulin A), or liver cirrhosis (elevated ╬│-globulin) are occasionally observed in patients with chronic liver disease [27]. Moreover, marked dyslipidemia is often observed in patients with liver disease. Hypertriglyceridemia can occur due to excessive alcohol consumption, interferon treatment, and uncontrolled diabetes mellitus; mixed dyslipidemia can occur due to hepatitis B virus- or C virus-related nephrotic syndrome; and hypercholesterolemia can occur due to primary biliary cirrhosis or other cholestatic liver diseases [50-53]. Consequently, the aforementioned liver diseases are potential causes of pseudohyponatremia. Pseudohyponatremia is commonly observed using indirect ion-selective electrodes for serum electrolyte measurement [19,54]. Therefore, the use of direct ion-selective electrodes or an osmometer for serum osmolality measurements is suggested to determine the true sNa concentration [19,54].
Symptomatic moderate or severe/profound hyponatremia should be treated promptly with hypertonic saline to improve symptoms, and this should be prioritized over further diagnostic testing [1,55]. If acute management has been initiated or there is asymptomatic or mild hyponatremia, step 3 should be followed.
The ECF status is divided into hypovolemia, euvolemia, or hypervolemia using the patientŌĆÖs history and physical examinations. A urine sodium level Ōēż30 mmol/L indicates low effective arterial volume, including hypovolemia and hypervolemia. For contracted ECF volume (hypovolemia), non-renal sodium loss including diarrhea, vomiting, or remote diuretics should be considered, while for expanded ECF volume (hypervolemia), liver cirrhosis, HF, and nephrotic syndrome should be considered. When the urine sodium level is >30 mmol/L, diuretics and kidney disease should be ruled out and ECF status should be assessed. For contracted ECF volume (hypovolemia), diuretics or renal sodium loss including renal or cerebral salt wasting should be considered, while for normal ECF volume (euvolemia), SIAD, secondary adrenal insufficiency, or hypothyroidism should be ruled out [1,27,55]. The clinical assessment to determine the volume status is difficult as both the sensitivity (0.5ŌĆō0.8) and specificity (0.3ŌĆō0.5) are low [1]. Therefore, additional modalities such as fractional excretion of sodium (with a cutoff of 1) or urea (with a cutoff of 35) or point-of-care ultrasound (POCUS) can be helpful for assessing volume status [56,57]. The fractional excretion of urea can provide additional assistance in patients receiving diuretics [57].
Hyponatremia has been linked to higher morbidity and mortality in patients with acute and chronic liver disease, acute-on-chronic liver failure (ACLF), or those awaiting LT [58,59].
Several studies have found that hyponatremia in liver cirrhosis is associated with a high incidence of liver-related complications, including a high prevalence of RA, HE, spontaneous bacterial peritonitis, and HRS; an increased need for massive paracentesis; and a shorter interval between paracenteses [3].
Patients with acute hyponatremia have a higher incidence of neurological symptoms (such as headache, disorientation, confusion, focal neurological deficits, seizures, and in some cases, death due to brain herniation) than patients with chronic hyponatremia [60]. Hypoosmolar hyponatremia expands astrocytes by moving water from the extracellular space into astrocytes to maintain osmotic balance; however, the expansion of brain cells is constricted due to the limited anatomical space [60,61]. Accordingly, intracellular solutes are discharged in the opposite direction to reduce intracellular osmotic pressure. Intracellular potassium is excreted first, followed by organic osmotic pressure, including myoinositol, glutamine, choline, and taurine shifts [60,61]. This protective adaptation mechanism can be observed in chronic hyponatremia and liver disease. The secondary increase in the intracellular glutamine content due to ammonia metabolism acts synergistically, mainly resulting in astrocyte edema and dysfunction of the glial-neuronal communication pathway [15,62,63]. Hyponatremia then further exacerbates astrocyte swelling and causes HE in patients with liver cirrhosis [4,15]. Hyponatremia is a major risk factor for HE in patients with liver cirrhosis, increasing HE risk by 8.36 times [4]. Therefore, monitoring sNa levels in patients with cirrhosis is critical for preventing or managing HE.
The pathogenesis of ascites is similar to that of hyponatremia due to excess water in the body, arterial vasodilation, arterial underfilling, and activation of the renin-angiotensin-aldosterone system and ADH [64-66]. In addition, a large amount of body fluid may accumulate due to increased intestinal capillary permeability resulting from the decrease in albumin synthesis caused by decreased liver function [66]. As much as 10% of patients with decompensated liver cirrhosis develop RA, characterized by severe ascites and requiring repeated large-volume paracenteses, even after receiving adequate diuretics and a salt-restricted diet [64-67]. One study found the incidence of RA to be higher in patients with hyponatremia than in patients with normal concentrations of sNa (sNa level <130 mmol/L: 29.4% vs. sNa level >135 mmol/L: 13.5%, p<0.001) [3]. A study of American veterans showed that the 1-year mortality rate for patients with RA was close to 70% [68]. Similarly, a study in Spain showed that the 1-year survival rate among patients with RA was only 31.6% and also showed that hyponatremia was an independent predictor of survival in patients with cirrhosis and RA [69].
Approximately 14ŌĆō50% of patients with ascites and hyponatremia develop renal dysfunction in the form of acute renal injury or HRS [44,69,70]. Renal failure is more common in patient s who have hy p onatremia with ascites, and hyponatremia is known to be an independent risk factor for acute renal injury (28% of those with sNa levels >135 mmol/L, 40% of those with sNa levels <130 mmol/L) [3].
ACLF is defined as acute and rapidly progressive liver failure in patients with previously diagnosed or undiagnosed chronic liver disease, associated with multi-organ failure and very high short-term mortality [59,71]. Approximately 25% of patients with ACLF have hyponatremia [59], and the frequency of hyponatremia is higher on the first day of hospitalization in patients with acutely decompensated cirrhosis and ACLF (without ACLF: 10.5% vs. with ACLF: 22.2%) [71]. In cirrhotic patients hospitalized with bacterial infection, the presence of hyponatremia is an independent predictor of ACLF and subsequent mortality [72].
A prospective cohort study showed that patients referred for consideration of LT with hyponatremia have a higher risk of early death regardless of cirrhosis severity, as assessed using the MELD score [5]. Therefore, some researchers have advocated for rapid LT in patients with cirrhosis and a MELD score <21, persistent ascites, and hyponatremia [5]. Another prospective multicenter study showed that incorporating the sNa level into the MELD score more accurately predicted survival compared to the MELD alone [73]. As a result of these studies, the United Network for Organ Sharing incorporated the sNa level into the MELD score (MELD-Na) for organ allocation in January 2016 [74].
The effect of pre-transplant hyponatremia on survival after transplantation is controversial. The largest study (n=19,537) conducted during the MELD era showed no significant reduction in survival after LT for patients with hyponatremia [75]. In another study conducted in the US, no significant difference in the survival rate at 90 days after LT between recipients with normal sNa concentrations and those with hyponatremia was found [76]. Another UK study revealed that the 3-year risk-adjusted mortality rate was higher in patients with pre-transplant hyponatremia than those with normal sNa levels [77]. A small single-center study also showed a relative decrease in 3-month survival in patients with an sNa concentration <130 mmol/L compared to patients without hyponatremia [78]. In addition, patients with hyponatremia had a high incidence of neurologic complications, renal failure, prolonged hospitalization in an intensive care unit, and increased ventilator requirements after LT [78]. The impact of pretransplant hyponatremia on posttransplant prognosis is still controversial; however, hyponatremia correction before transplantation is one of the methods used to reduce the risk of various complications.
Osmotic demyelination syndrome (ODS) is a rare but irreversible neurologic complication from excessive hyponatremia therapy that can result in death. When sNa levels return to normal, electrolytes and osmolality are restored in brain cells. Electrolytes are rapidly corrected, whereas organic osmolality is corrected slowly. This results in the shrinkage of glial cells, axonal shear damage, tight junction destruction, and cell death [79]. ODS occurrence is difficult to predict [80]; however, it has been established that individuals who are alcoholics, malnourished, or have advanced liver disease and are liver transplant recipients are at high risk for developing ODS, especially during overcorrection of chronic hyponatremia [27,81]. Furthermore, the conditions that increase susceptibility to ODS, including malnutrition, hypokalemia, and hypoxia, are frequently observed in patients with liver disease [27,81]. ODS symptoms can range from mild behavioral changes to dysarthria, dysphagia, ataxia, ParkinsonŌĆÖs disease, and widespread neurological deficits. According to the 2013 American guidelines, patients with advanced liver disease are classified as high risk for developing ODS; thus, a lower target of 4ŌĆō6 mmol/L/day, with a maximum of 8 mmol/L in any 24-hour period, is recommended [81]. Therefore, assessing the underlying disease and major risk factors for ODS is critical for the proper management of hyponatremia in patients with liver disease.
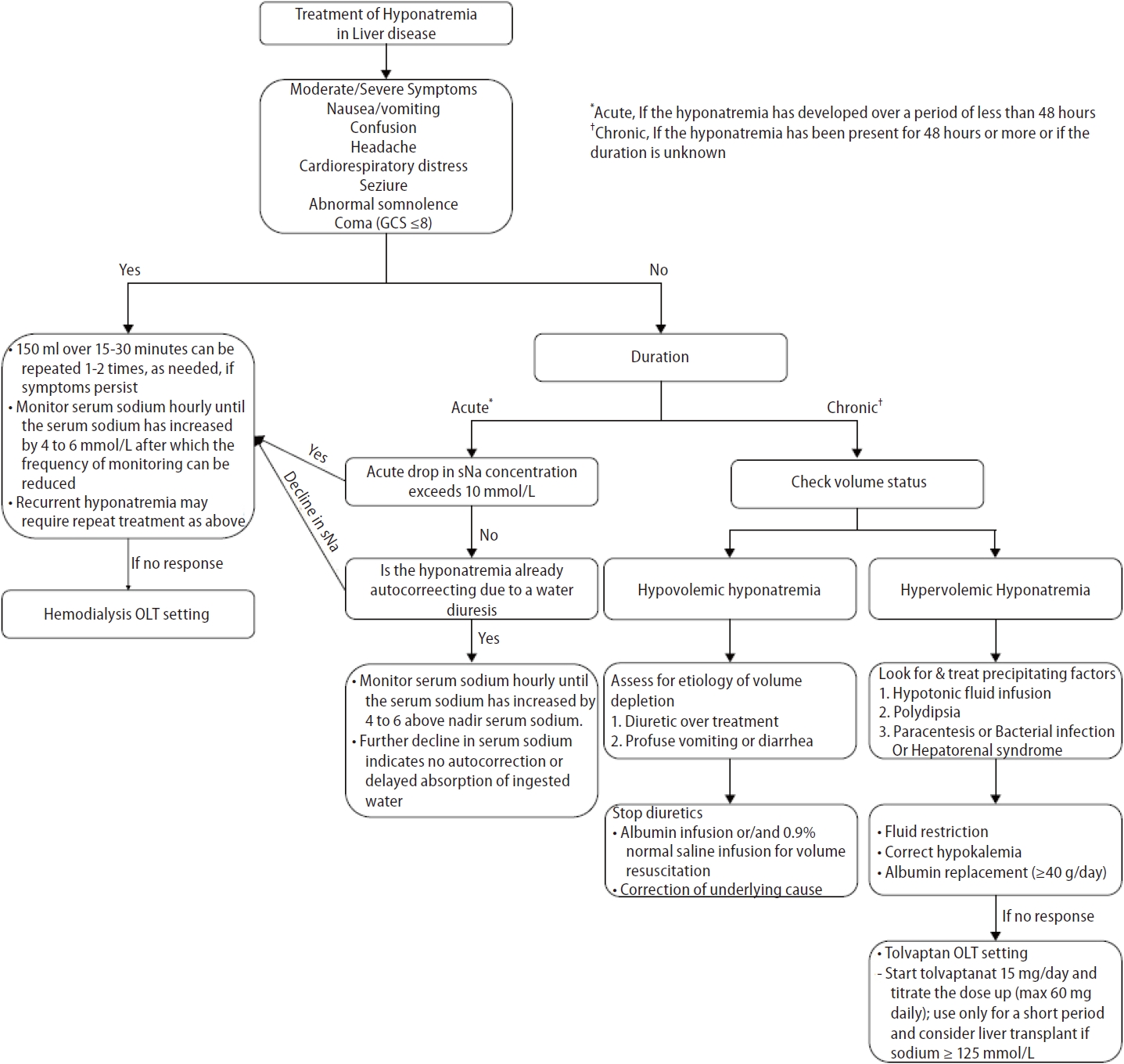
The approach to hyponatremia treatment in liver disease is similar to that used in the general population; however, some specific considerations are required in advanced liver disease. Hyponatremia management is guided by the severity of symptoms (asymptomatic or symptomatic [mild, moderate, severe]) and duration of hyponatremia (acute [<48 h] or chronic [Ōēź48 h or unknown]). Hyponatremia treatment aims to prevent a further decrease in the sNa concentration, reduce intracranial pressure in patients at risk of brain prolapse, relieve hyponatremia symptoms, and prevent overcorrection. Hyponatremia management strategies in patients with liver disease are presented in Figure 3.
The 2013 American guidelines [81] and 2014 European guidelines [1] recommend that patients with moderately or severely symptomatic hyponatremia undergo treatment with hypertonic saline. In cases of severe symptomatic hyponatremia, rapid intermittent bolus regimens of hypertonic saline should be immediately infused to raise sNa levels by 4 to 6 mmol/L to improve cerebral edema. In cases of moderate symptomatic hyponatremia, rapid intermittent boluses or slow continuous infusions of hypertonic saline may be administered. The target correction rate is an sNa concentration of 5ŌĆō9 mmol/L within the first 24 hours and 10ŌĆō17 mmol/L or 130 mmol/L within 48 hours [82]. However, for patients with decompensated liver cirrhosis, administration of hypertonic saline should be limited to severely symptomatic patients with life-threatening conditions such as cardio-respiratory distress, seizures, and coma because it may worsen volume overload or increases ascites and edema. Hypertonic saline is also used to increase sNa levels in patients with hyponatremia before LT to prevent rapid hyponatremia correction after LT [83]. The correction rate for hyponatremia should not exceed 8 mmol/L in any 24-h period in advanced liver disease because of the high risk for ODS [81].
An absence of symptoms indicates that significant brain swelling has not yet occurred. Therefore, instead of administering hypertonic saline infusions, the etiology of hyponatremia must be assessed and cause-specific treatments be initiated. However, if the acute drop in sNa concentration exceeds 10 mmol/L, hypertonic saline should be administered to prevent a further decrease in the concentration [1]. Hypertonic saline administration may be delayed if hyponatremia is corrected by water diuresis. Urine volume, low urine specific gravity, and low urine osmolality can help clinicians determine the hypertonic saline infusion volume. The treatment goal is to rapidly increase the sNa concentration to 4ŌĆō6 mmol/L to relieve symptoms and prevent brain hernias [1].
Asymptomatic chronic hyponatremia does not require urgent correction; however, identifying and treating the underlying cause is critical. In liver cirrhosis, hyponatremia is mostly the chronic asymptomatic type due to brain adaptation. Therefore, it is essential to differentiate the cause of hyponatremia based on the ECF volume status.
Hypovolemic hyponatremia is caused by excessive use of diuretics and reduced effective volume due to gastrointestinal bleeding. Thus, hypovolemic hyponatremia must be corrected by discontinuing the diuretics and expanding the plasma volume using crystalloid fluids, albumin, etc.
Hypervolemic or dilutive hyponatremia is common and worsens as liver disease advances. Considering the etiology of hypervolemic hyponatremia, the ideal solution is to increase the excretion of water without solutes. Various management methods have been introduced and are discussed below. The treatment evidence is summarized in Table 2.
Fluid restriction has been suggested as a first-line treatment for hyponatremia. For effective fluid restriction, water intake should be about 500 mL/day less than the sum of urine output and insensible loss (1ŌĆō1.5 L/day) [14]. Fluid restriction should be considered if there are neurological symptoms or if the sNa concentration is <120 mmol/L; however, the role of fluid restriction is limited in patients with mild, asymptomatic hyponatremia [3]. The water restriction method is associated with poor patient compliance, making it difficult to achieve sNa normalization. A good indicator of adequate fluid restriction is a change in the sNa concentration within the first 24ŌĆō48 hours. However, if sNa levels do not increase within the first 48ŌĆō72 hours, other options should be considered [84].
Hypokalemia may result from increased urine loss due to diuretic treatment or increased gastrointestinal fluid loss in patients with diarrhea or vomiting. Hypokalemia correction is recommended for two reasons: hypokalemia increases renal ammonia synthesis, and concomitant alkalosis increases the fraction of bound ammonia in plasma, which can lead to HE. When osmotically active potassium is corrected, replenished potassium enters the cells, and intracellular sodium moves in the opposite direction, increasing the sNa concentration and osmotic pressure without external sodium administration [45,47]. Hence, since potassium is as osmotically active as sodium, excessive sNa and hypokalemia corrections can cause severe complications such as ODS due to overcorrection [45].
Key question: In hyponatremic patients with liver disease, does the vaptan group show more effective sodium correction than the no vaptan group?
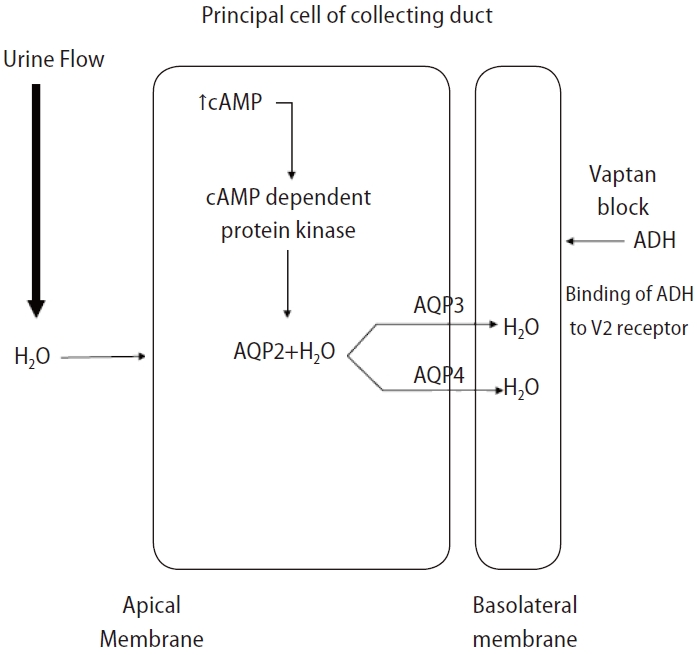
Vasopressin receptor antagonists or vaptans are non-peptide drugs that inhibit ADH action on the V1a, V1b, and V2 receptors [85]. The V2 receptor responsible for water absorption is located in the basolateral membrane of the renal collecting duct, and a blockade of this receptor can cause water diuresis and increase the sNa concentration (Fig. 4) [86]. The V2 selective blocker of ADH effectively improves the sNa concentration in SIAD or HF cases with elevated vasopressin [87].
Tolvaptan is the only selective V2 receptor antagonist that can be administered orally, and several studies have shown that tolvaptan significantly increases the sNa concentration and improves ascites in patients with liver cirrhosis, but does not improve the survival rate. In a multicenter clinical trial that followed patients with HF, SIAD, and liver cirrhosis longitudinally for an average of 1.9 years, the sNa concentration significantly increased in patients with HF, SIAD, and liver cirrhosis after taking tolvaptan; however, the increase in the sNa concentration in patients with liver cirrhosis was slower than that in patients with HF and SIAD [88]. To evaluate the effect of tolvaptan in terms of sodium correction, we performed a database search of Ovid MEDLINE and conducted a meta-analysis of existing randomized controlled trials (Fig. 5A). Tolvaptan was found to be effective at correcting hyponatremia in patients with liver disease (Fig. 5B and Table 2) [89-93]. Additionally, the beneficial effect of vaptan on ascites was confirmed in three meta-analyses [94-96]. A recent cohort study showed that an increase in sNa levels after one month of tolvaptan treatment could positively affect the risk of death in patients with cirrhosis and hyponatremia [97]. However, a previous meta-analyses reported no change in the short-term and long-term survival of patients with cirrhosis after treatment with vaptan [94-96]. For patients with cirrhosis and hyponatremia at baseline, survival rates tended to improve after treatment with vaptan, though it was not statistically significant [96]. The most common side effects of tolvaptan include thirst, polyuria, and dry mouth. Serious side effects such as dehydration with hypotension, dehydration with dizziness, syncope, and acute renal failure can also occur. In an open-label phase 4 study aimed at evaluating the efficacy and safety of tolvaptan in autosomal dominant polycystic kidney disease, aspartate transaminase and alanine transaminase levels were elevated in two patients (1.7%), and these abnormal findings improved after maintaining the dose or temporarily discontinuing tolvaptan [98]. Therefore, the US Food & Drug Administration has limited tolvaptan use to less than 30 days and recommends that it not be used in patients with underlying cirrhosis due to the risk of liver failure and death [99]. However, it should be noted that the dose of tolvaptan in this study was 4ŌĆō8 times the dose commonly used to treat hyponatremia [100]. The European Association for the Study of the Liver clinical practice guidelines for decompensated cirrhosis recommends the routine use of tolvaptan for correction of low sodium levels only in controlled clinical trials [101]. KoreaŌĆÖs Ministry of Food and Drug Safety has approved tolvaptan for hyponatremic patients with HF or SIAD, but not for patients with liver disease [102]. The Japanese Society of Gastroenterology does not mention tolvaptan use for correcting hyponatremia; however, tolvaptan has been approved as an additional drug for fluid retention in patients with liver cirrhosis and is recommended as an additional drug for patients with ascites that is refractory to treatment with existing diuretics (furosemide and spironolactone) [103]. The use of vaptans in patients with reduced liver function is restricted in most countries, and caution is advised when administering vaptans because relevant studies on long-term survival are lacking.
Key question: In hyponatremic patients with liver disease, does the albumin group show more effective sodium correction than the no albumin group?
Albumin replacement is another treatment option that can improve hyponatremia associated with liver cirrhosis. Furthermore, albumin replacement in patients with cirrhosis has been widely used to manage various complications such as spontaneous bacterial peritonitis, HRS, ascites, and HE [104-109]. Previous authors have argued that intravenous albumin infusions can increase the sNa concentration through expanding the intravascular volume, thus increasing free water clearance in the urine [102,110]. Regarding the pathogenesis of hyponatremia in liver cirrhosis, the sNa concentration can be increased by maintaining colloidal osmotic pressure and affecting the inflammatory pathway, improving the hypervolemic circulation state, and removing inflammatory factors [111].
Most previous studies have evaluated the role that albumin replacement plays in preventing hyponatremia after largevolume paracentesis in cirrhosis hyponatremia, but relatively few studies have evaluated the role that albumin plays in hyponatremia treatment [112-117]. In a retrospective multicenter study of 1,125 patients with liver cirrhosis accompanied by hyponatremia (regardless of large-volume paracentesis) at the time of admission, the delta values of the sNa concentrations between the albumin replacement group and the nonalbumin replacement group were compared, showing significantly higher delta values in the albumin replacement group [113]. In addition, the post-hoc analysis of the ANSWER study, which compared the group receiving long-term albumin treatment (20% human albumin in a 50 mL vial: 40 g twice a week for 2 weeks, then 40 g weekly thereafter) to the group receiving standard medical treatment, showed at the 3-month follow-up that albumin was associated with sNa normalization over time [115]. The most recent meta-analysis evaluating the efficacy of albumin replacement also suggested that albumin replacement can be helpful in the prevention and treatment of hyponatremia in liver cirrhosis [116]. However, recommendations on the use of albumin replacement therapy in the management of cirrhosis with hyponatremia vary according to clinical guideline. While some suggest that albumin should be used to prevent or treat hyponatremia in liver cirrhosis [103], other reports recommend that albumin infusions be carefully evaluated due to lack of data [111,118].
We searched the Ovid MEDLINE database for studies evaluating the efficacy of albumin infusions for sodium correction in patients with liver disease. Only one RCT and two cohort studies were found that evaluated the use of albumin in the treatment of hyponatremia for patients with liver cirrhosis. The results of this one RCT are replicated here, and we performed a meta-analysis of the two cohort studies (Fig. 5C and Table 2) [113,115,117]. Our analysis (Fig. 5D) showed that the use of albumin was significant for treating hyponatremia that had already occurred. In most studies, the use of albumin resulted in significant improvements in hyponatremia; however, longterm outcomes, including the survival rate, showed a heterogeneous relationship. In a cohort study conducted by Bajaj et al. [113] (2018) that evaluated 1,126 patients with hyponatremia, the albumin group showed resolution of hyponatremia and a higher 30-day survival rate. However, a post hoc analysis of the ATTIRE trial conducted in 2021, which included 206 hyponatremic patients, showed that albumin infusions were not associated with mortality [114]. In another cohort study conducted by Bai et al. [117] (2022), which included 339 patients with liver cirrhosis, albumin infusions were found to effectively improve serum sodium levels during hospitalization in patients with cirrhosis and hyponatremia, but did not have a significant effect on long-term mortality.
Although high-quality studies on hyponatremia treatment are lacking, it appears that albumin injections may help prevent and treat hyponatremia in patients with cirrhosis. However, these findings must be validated through a well-designed, large-scale study, and the optimal dosages and durations of treatment and their effect on outcomes, including survival rates, must be further evaluated.
Pre-transplant hyponatremia is associated with prolonged hospitalization, prolonged intensive care unit admission, and neurologic complications [76-78,119,120]. Efforts to optimize sNa levels to >125 mmol/L before LT are needed, as patients with severe hyponatremia before transplantation are more prone to neurological complications such as ODS after transplantation [76,121,122]. The risk of ODS after LT in patients with liver disease increases with lower baseline sNa levels [76]. Patients undergoing LT are at increased risk for rapid sNa correction due to fluid shifts that occur during surgery from the intraoperative administration of intravenous crystalloids, blood products, and sodium bicarbonate [10,83,123]. However, despite the importance of correcting hyponatremia, no definitive protocol for perioperative management of hyponatremia and no correction threshold for sNa levels are currently available [123].
The incidence of ODS, a severe complication caused by rapid sNa correction after LT, ranges from 0.5% to 29% [7,76,124]. Symptoms such as quadriplegia (up to 45%) and seizures (27ŌĆō36%) occur within 1ŌĆō2 weeks after LT [7,125,126]. Since ODS is associated with poor prognosis [76,125,126], various methods have been used to prevent rapid correction of sNa. LT recipients receive a large amount of sodium-containing intravenous fluids, such as packed red blood cells, platelet concentrates, and fresh frozen plasma; thus rapid sNa correction may occur after surgery [10,83,123]. To minimize this rapid correction, 0.45% saline or 5% glucose water can be used instead of 0.9% normal saline [127]. Additionally, prothrombin complex concentrates with low sNa levels can be used instead of fresh frozen plasma [127]. Finally, for patients who have received or will receive high intraoperative sodium loads through blood transfusions and intravenous fluids, continuous veno-venous hemofiltration using dilute solutions before or during surgery may be considered for managing rapid changes in sNa levels [123]; however, relevant data are very limited.
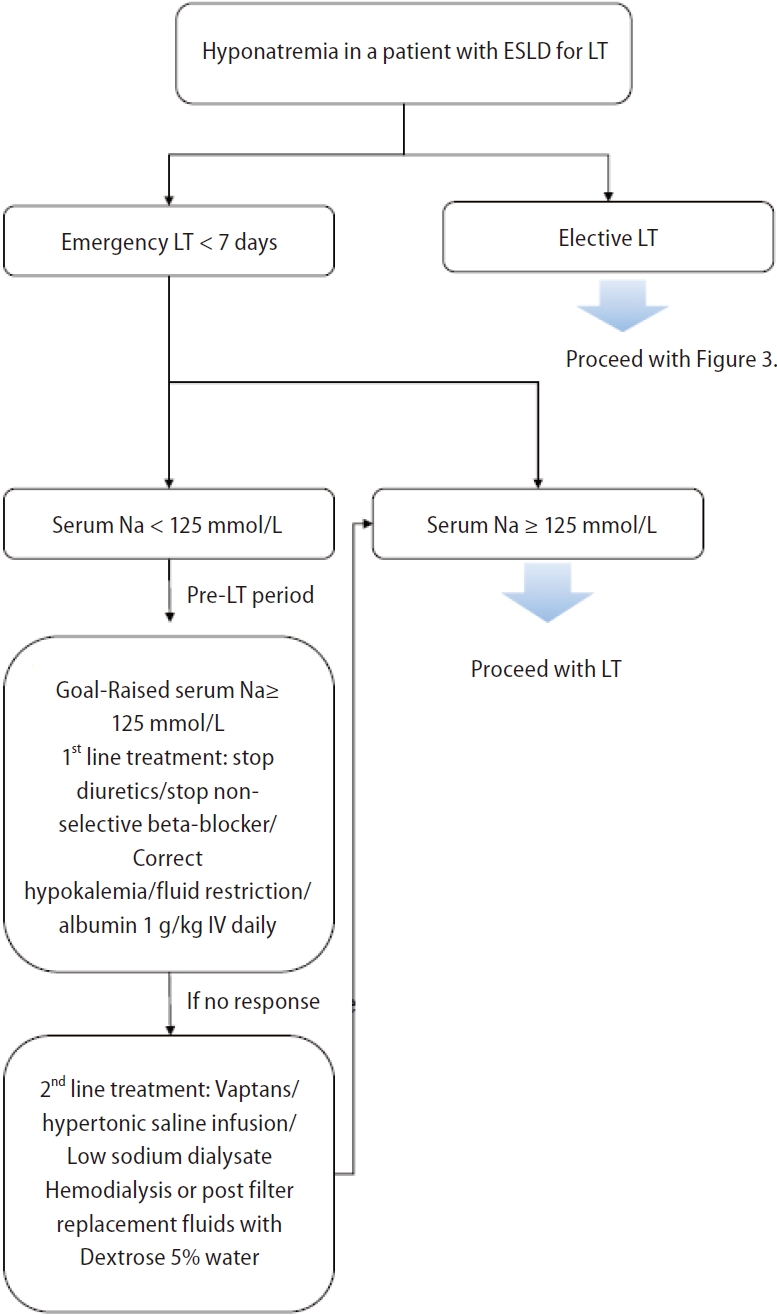
Since hyponatremia improves when fundamental liver function is restored after LT, the occurrence of severe complications such as ODS due to rapid changes in sNa must be monitored. Some studies have shown that mortality due to ODS after LT is 40% at 3 months [126] and 63% at 1 year [76], and up to 84% of surviving patients have been reported to suffer from permanent sequelae [125,126]. Desmopressin administration as a rescue measure is useful when unintended overcorrection of hyponatremia occurs [128]. One proposed approach for the perioperative management of hyponatremia in patients awaiting LT is summarized in Figure 6 [129].
Hyponatremia is a pervasive electrolyte disorder in advanced chronic liver disease. Fundamental treatment strategies include water restriction, hypokalemia correction, and diuretic discontinuation. Hypertonic saline can be considered in acutely symptomatic hyponatremia; however, caution is advised given the potential for fatal complications such as ODS and worsening ascites. Furthermore, although vasopressin receptor antagonists and albumin replacement can be used to correct hyponatremia in liver cirrhosis, supportive studies are lacking, and individual countries may have controversies regarding their use. Although LT is ideal for managing hyponatremia in patients with advanced chronic liver disease, multidisciplinary approaches to perioperative management are essential for correcting hyponatremia.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Research Foundation of Korea (no. 2021R1C1C1008966).
FOOTNOTES
Figure┬Ā1.
Pathogenesis of hypervolemic hyponatremia in patients with liver cirrhosis. ADH, antidiuretic hormone; DNA, deoxyribonucleic acid; NO, nitric oxide; PG, prostaglandin; RES, reticuloendothelial system.
Figure┬Ā2.
Hyponatremia diagnostic approach in patients with liver disease. CHF, congestive heart failure; GI, gastrointestinal; LVP, large volume paracentesis; SIAD, syndrome of inappropriate antidiuresis.
Figure┬Ā3.
Treatment of hyponatremia in patients with liver disease. GCS, Glasgow Coma Scale; OLT, orthotopic liver transplant; sNa, serum sodium concentration. *Acute, If the hyponatremia has developed over a period of less than 48 hours. ŌĆĀChronic, If the hyponatremia has been present for 48 hours or more or if the duration is unknown.
Figure┬Ā4.
Mechanism of action of vaptan. ADH, antidiuretic hormone; AQP2, aquaporin-2; AQP3, aquaporin-3; AQP4, aquaporin-4.
Figure┬Ā5.
Results of hyponatremia treatment in liver disease. (A) Flowchart of selection of studies on the effect of tolvaptan treatment on hyponatremia. (B) Forest plot showing the effect of tolvaptan treatment on hyponatremia (change from baseline hyponatremia [mmol/L]). (C) Flowchart of selection of studies on the effect of albumin treatment on hyponatremia. (D) Forest plot showing the effect of albumin treatment on hyponatremia (improvement in hyponatremia [%]).

Figure┬Ā6.
Management of a patient with hyponatremia and end-stage liver disease (ESLD) for liver transplantation (LT) (Figure modified from Praharaj and Anand. J Clin Exp Hepatol 2022;12:575-594) [129].
Table┬Ā1.
Classification on hyponatremia in liver disease
| Classification | ||
|---|---|---|
| Serum sodium concentration (I) | (II)ŌĆĀ | |
| ŌĆāMild | 130ŌĆō134 mmol/L | 125ŌĆō129 mmol/L |
| ŌĆāModerate | 125ŌĆō129 mmol/L | 120ŌĆō124 mmol/L |
| ŌĆāSevere | <125 mmol/L | <120 mmol/L |
| Severity of clinical symptoms | ||
| ŌĆāAsymptomatic-mild | Less pronounced | |
| ŌĆāModerate | Nausea without vomiting, confusion, headache, drowsiness, general weakness, myalgia | |
| ŌĆāSevere | Vomiting, stupor, seizures, coma (Glasgow Coma Scale Ōēż8) | |
| Time of development | ||
| ŌĆāAcute | <48 hours | |
| ŌĆāChronic | Ōēź48 hours | |
| Serum osmolality | ||
| ŌĆāHypotonic | <275 mOsm/kg | |
| ŌĆāIsotonic (pseudohyponatremia) | 275ŌĆō295 mOsm/kg | |
| ŌĆāHypertonic | >295 mOsm/kg | |
| Clinical assessment of volume status | ||
| ŌĆāHypervolemic | Ascites, pedal edema, anasarca | 90% |
| ŌĆāEuvolemic | Rare | |
| ŌĆāHypovolemic | No ascites/pedal edema | 10% |
Table┬Ā2.
Characteristics of the studied for the treatment of hyponatremia in liver disease according to use of tolvaptan or albumin infusion
| First author (yr) | Study design | Sample Size (n) | Intervention group (n) | Control group (n) | Definition of hyponatremia | Dose | Control group | Result | |
|---|---|---|---|---|---|---|---|---|---|
| Tolvaptan | |||||||||
| Schrier et al. [89] (2006) | RCT | 448 | 225 | 223 | sNa <135 mmol/L | Tolvaptan 15, 30, 60 mg/d | Placebo | See Figure 5B | |
| C├Īrdenas et al. [90] (2012) | RCT | 120 | 63 | 57 | sNa <135 mmol/L | Tolvaptan 15, 30, 60 mg/d | Placebo | ||
| Rai et al. [91] (2017) | RCT | 50 | 25 | 25 | NA | Tolvaptan 7.5, 15 mg/d | Midodrine SMT | ||
| Wang et al. [92] (2018) | RCT | 98 | 69 | 29 | sNa <135 mmol/L | Tolvaptan 7.5, 15, 30 mg/d | Placebo | ||
| Tang et al. [93] (2020) | RCT | 143 | 126 | 17 | sNa <135 mmol/L | Tolvaptan 7.5, 15 mg/d | Placebo | ||
| Albumin infusion | |||||||||
| Bajaj et al. [107] (2018) | Cohort | 1,121 | 774 | 347 | sNa Ōēż130 mmol/L | 225 g (IQR 100, 400g) | NA | See Figure 5D | |
| Bai et al. [117] (2022) | Cohort | 394 | 197 | 197 | sNa <135 mmol/L | 40 g (IQR 10,380g) | NA | ||
| Zaccherini et al. [109] (2023) | RCT | 149 | 60 | 89 | sNa <135 mmol/L | 20% HA in 50-mL vials: 40 g twice a week for 2 weeks and then, 40 g weekly | SMT | Long-term albumin administration improves hyponatremia and reduces episodes of at least moderate hyponatremia. | |
REFERENCES
1. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al.; Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014;170:G1-G47. Eur J Endocrinol 2014;170:G1-G47 Erratum in: Eur J Endocrinol 2014;171:X1.

2. Gin├®s P, Berl T, Bernardi M, Bichet DG, Hamon G, Jim├®nez W, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 1998;28:851-864.


3. Angeli P, Wong F, Watson H, Gin├©s P; CAPPS Investigators. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006;44:1535-1542.


4. Guevara M, Baccaro ME, Torre A, G├│mez-Ans├│n B, R├Łos J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with timedependent analysis. Am J Gastroenterol 2009;104:1382-1389.



5. Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004;40:802-810.


6. Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis 2000;32:605-610.


7. Crivellin C, Cagnin A, Manara R, Boccagni P, Cillo U, Feltracco P, et al. Risk factors for central pontine and extrapontine myelinolysis after liver transplantation: a single-center study. Transplantation 2015;99:1257-1264.

8. Forns X, Gin├©s A, Gin├©s P, Arroyo V. Management of ascites and renal failure in cirrhosis. Semin Liver Dis 1994;14:82-96.


9. Abelmann WH. Hyperdynamic circulation in cirrhosis: a historical perspective. Hepatology 1994;20:1356-1358.


10. Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology 1994;20:1359-1363.


11. Gin├©s P, Fern├Īndez-Esparrach G, Arroyo V, Rod├®s J. Pathogenesis of ascites in cirrhosis. Semin Liver Dis 1997;17:175-189.


13. Martell M, Coll M, Ezkurdia N, Raurell I, Genesc├Ā J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol 2010;2:208-220.



14. John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol 2015;21:3197-3205.



15. Gin├©s P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 2008;48:1002-1010.


16. Asbert M, Gin├©s A, Gin├©s P, Jim├®nez W, Cl├Āria J, Sal├│ J, et al. Circulating levels of endothelin in cirrhosis. Gastroenterology 1993;104:1485-1491.


17. Henriksen JH, Bendtsen F, Gerbes AL, Christensen NJ, RingLarsen H, S├Ėrensen TI. Estimated central blood volume in cirrhosis: relationship to sympathetic nervous activity, betaadrenergic blockade and atrial natriuretic factor. Hepatology 1992;16:1163-1170.


18. Arroyo V, Bosch J, Gaya-Beltr├Īn J, Kravetz D, Estrada L, Rivera F, et al. Plasma renin activity and urinary sodium excretion as prognostic indicators in nonazotemic cirrhosis with ascites. Ann Intern Med 1981;94:198-201.


19. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013;38:50-57.



20. Schrier RW. Diseases of the Kidney & Urinary Tract. Vol. 3. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2006.
21. Knepper MA, Wade JB, Terris J, Ecelbarger CA, Marples D, Mandon B, et al. Renal aquaporins. Kidney Int 1996;49:1712-1717.


22. Nielsen S, Marples D, Fr├Ėkiaer J, Knepper M, Agre P. The aquaporin family of water channels in kidney: an update on physiology and pathophysiology of aquaporin-2. Kidney Int 1996;49:1718-1723.


23. King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol 1996;58:619-648.


24. Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol 1997;272(1 Pt 2):F3-F12.


25. Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 1993;361:549-552.



26. Agre P, Nielsen S. The aquaporin family of water channels in kidney. Nephrologie 1996;17:409-415.

27. Liamis G, Filippatos TD, Liontos A, Elisaf MS. Hyponatremia in patients with liver diseases: not just a cirrhosis-induced hemodynamic compromise. Hepatol Int 2016;10:762-772.



28. Bozanich NK, Kwo PY. Renal insufficiency in the patient with chronic liver disease. Clin Liver Dis 2015;19:45-56.


29. Qureshi MO, Shafqat F, Dar FS, Salih M, Khokhar N. Renal failure in patients with end stage liver disease and its impact on clinical outcome. J Coll Physicians Surg Pak 2014;24:628-631.

30. Liamis G, Milionis HJ, Elisaf M. Hyponatremia in patients with infectious diseases. J Infect 2011;63:327-335.


31. Gupta A, Quigg RJ. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis 2015;22:343-351.


32. Filippatos TD, Elisaf MS. Hyponatremia in patients with heart failure. World J Cardiol 2013;5:317-328.



33. Handler J. Well tolerated spironolactone-related hyponatremia. J Clin Hypertens (Greenwich) 2008;10:317-321.



34. Yim SY, Seo YS, Jung CH, Kim TH, Kim ES, Keum B, et al. Risk factors for developing hyponatremia during terlipressin treatment: a retrospective analyses in variceal bleeding. J Clin Gastroenterol 2015;49:607-612.

35. Han X, Li J, Yang JM, Gao M, Wang L. A retrospective analysis of hyponatremia during terlipressin treatment in patients with esophageal or gastric variceal bleeding due to portal hypertension. JGH Open 2019;4:368-370.




36. Xu X, Lin S, Yang Y, Chen Y, Liu B, Li B, et al. Development of hyponatremia after terlipressin in cirrhotic patients with acute gastrointestinal bleeding: a retrospective multicenter observational study. Expert Opin Drug Saf 2020;19:641-647.


37. Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-3524.


38. Abou-Alfa GK, Capanu M, OŌĆÖReilly EM, Ma J, Chou JF, Gansukh B, et al. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol 2014;60:319-324.



39. Hedrick KT, Just SM, Kahn DR. Probable boceprevir-induced hyponatremia in a patient with chronic hepatitis C. Am J Health Syst Pharm 2015;72:449-452.


40. Arieff AI, Llach F, Massry SG. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine (Baltimore) 1976;55:121-129.


41. Schrier RW. Body water homeostasis: clinical disorders of urinary dilution and concentration. J Am Soc Nephrol 2006;17:1820-1832.

44. Gin├©s A, Escorsell A, Gin├©s P, Sal├│ J, Jim├®nez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993;105:229-236.


45. Rose BD, Post TW. Clinical Physiology of Acid-Base and Electrolyte Disorders. 5th ed. New York: McGraw-Hill, 2001.
46. Yeates KE, Singer M, Morton AR. Salt and water: a simple approach to hyponatremia. CMAJ 2004;170:365-369. CMAJ 2004;170:365-369 Erratum in: CMAJ 2004;170:931.


47. Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 2007;120(11 Suppl 1):S1-S21.


48. Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med 1999;106:399-403.


49. Katz MA. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med 1973;289:843-844.


50. Filippatos TD, Elisaf MS. Recommendations for severe hypertriglyceridemia treatment, are there new strategies? Curr Vasc Pharmacol 2014;12:598-616.


51. Hussain I, Ahmad Z, Garg A. Extreme hypercholesterolemia presenting with pseudohyponatremia - a case report and review of the literature. J Clin Lipidol 2015;9:260-264.


52. Garibaldi BT, Cameron SJ, Choi M. Pseudohyponatremia in a patient with HIV and hepatitis C coinfection. J Gen Intern Med 2008;23:202-205.




53. Farooqi MS, Hashim IA. A woman with primary biliary cirrhosis and hyponatremia. Clin Chem 2015;61:1028-1031.



54. Filippatos TD, Liamis G, Christopoulou F, Elisaf MS. Ten common pitfalls in the evaluation of patients with hyponatremia. Eur J Intern Med 2016;29:22-25.


55. Lee Y, Yoo KD, Baek SH, Kim YG, Kim HJ, Ryu JY, et al. Korean Society of Nephrology 2022 Recommendations on controversial issues in diagnosis and management of hyponatremia. Kidney Res Clin Pract 2022;41:393-411.




56. Rondon-Berrios H, Berl T. Vasopressin receptor antagonists in hyponatremia: uses and misuses. Front Med (Lausanne) 2017;4:141.



57. Jim├®nez JV, Carrillo-P├®rez DL, Rosado-Canto R, Garc├Ła-Ju├Īrez I, Torre A, Kershenobich D, et al. Electrolyte and acid-base disturbances in end-stage liver disease: a physiopathological approach. Dig Dis Sci 2017;62:1855-1871.



58. Murthy KA, Khan IM, Kiran PK, Hakeem H. A study of viral hepatitis e infection in a tertiary care hospital in mysore, South India. Open Forum Infect Dis 2014;1:ofu036.




59. C├Īrdenas A, Sol├Ā E, Rodr├Łguez E, Barreto R, Graupera I, Pavesi M, et al.; CANONIC study investigators of the EASL-CLIF Consortium. Hyponatremia influences the outcome of patients with acute-on-chronic liver failure: an analysis of the CANONIC study. Crit Care 2014;18:700.


60. Sterns RH, Silver SM. Brain volume regulation in response to hypo-osmolality and its correction. Am J Med 2006;119(7 Suppl 1):S12-S16.


61. Videen JS, Michaelis T, Pinto P, Ross BD. Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest 1995;95:788-793.



62. H├żussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006;43:1187-1190.


63. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol 2020;26:83-127.


64. Arroyo V, Gin├©s P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23:164-176.


65. Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int 2010;30:937-947.


66. Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World J Hepatol 2013;5:251-263.



67. Fortune B, Cardenas A. Ascites, refractory ascites and hyponatremia in cirrhosis. Gastroenterol Rep (Oxf) 2017;5:104-112.



68. Stanley MM, Ochi S, Lee KK, Nemchausky BA, Greenlee HB, Allen JI, et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with Ascites. N Engl J Med 1989;321:1632-1638.


69. Planas R, Montoliu S, Ballest├® B, Rivera M, Miquel M, Masnou H, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006;4:1385-1394.


70. Russ KB, Stevens TM, Singal AK. Acute kidney injury in patients with cirrhosis. J Clin Transl Hepatol 2015;3:195-204.



71. Pereira G, Baldin C, Piedade J, Reis V, Valdeolivas T, Victor L, et al. Combination and sequential evaluation of acute-onchronic liver failure (ACLF) and hyponatremia and prognosis in cirrhotic patients. Dig Liver Dis 2020;52:91-97.


72. Lopes-Secundo TM, Sev├Ī-Pereira T, Correa BR, Silva NCM, Imbrizi MR, Cunha-Silva M, et al. Serum sodium, model for endstage liver disease, and a recent invasive procedure are risk factors for severe acute-on-chronic liver failure and death in cirrhotic patients hospitalized with bacterial infection. Eur J Gastroenterol Hepatol 2018;30:1055-1059.

73. Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130:1652-1660.


74. Organ Procurement & Transplantation Network (OPTN). Liver policy. OPTN web site, <https://optn.transplant.hrsa.gov/governance/policy-initiatives/liver/>. Accessed 3 Mar 2023.
75. Leise MD, Yun BC, Larson JJ, Benson JT, Yang JD, Therneau TM, et al. Effect of the pretransplant serum sodium concentration on outcomes following liver transplantation. Liver Transpl 2014;20:687-697.



76. Yun BC, Kim WR, Benson JT, Biggins SW, Therneau TM, Kremers WK, et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology 2009;49:1610-1615.



77. Dawwas MF, Lewsey JD, Neuberger JM, Gimson AE. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl 2007;13:1115-1124.


78. Londo├▒o MC, Guevara M, Rimola A, Navasa M, Taur├Ā P, Mas A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology 2006;130:1135-1143.


80. Go S, Kim S, Son HE, Ryu JY, Yang H, Choi SR, et al. Association between copeptin levels and treatment responses to hypertonic saline infusion in patients with symptomatic hyponatremia: a prospective cohort study. Kidney Res Clin Pract 2021;40:371-382.




81. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013;126(10 Suppl 1):S1-S42.


82. Baek SH, Jo YH, Ahn S, Medina-Liabres K, Oh YK, Lee JB, et al. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: The SALSA randomized clinical trial. JAMA Intern Med 2021;181:81-92.



83. Crismale JF, Meliambro KA, DeMaria S Jr, Bronster DB, Florman S, Schiano TD. Prevention of the osmotic demyelination syndrome after liver transplantation: a multidisciplinary perspective. Am J Transplant 2017;17:2537-2545.



84. Sigal SH, Amin A, Chiodo JA 3rd, Sanyal A. Management strategies and outcomes for hyponatremia in cirrhosis in the hyponatremia registry. Can J Gastroenterol Hepatol 2018;2018:1579508.




85. Veeraveedu PT, Palaniyandi SS, Yamaguchi K, Komai Y, Thandavarayan RA, Sukumaran V, et al. Arginine vasopressin receptor antagonists (vaptans): pharmacological tools and potential therapeutic agents. Drug Discov Today 2010;15:826-841.


86. Holmes CL, Landry DW, Granton JT. Science review: vasopressin and the cardiovascular system part 1--receptor physiology. Crit Care 2003;7:427-434.



87. Quittnat F, Gross P. Vaptans and the treatment of water-retaining disorders. Semin Nephrol 2006;26:234-243.


88. Pose E, Sol├Ā E, Piano S, Gola E, Graupera I, Guevara M, et al. Limited efficacy of tolvaptan in patients with cirrhosis and severe hyponatremia: real-life experience. Am J Med 2017;130:372-375.


89. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099-2112.


90. C├Īrdenas A, Gin├©s P, Marotta P, Czerwiec F, Oyuang J, Guevara M, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol 2012;56:571-578.


91. Rai N, Singh B, Singh A, Vijayvergiya R, Sharma N, Bhalla A, et al. Midodrine and tolvaptan in patients with cirrhosis and refractory or recurrent ascites: a randomised pilot study. Liver Int 2017;37:406-414.



92. Wang S, Zhang X, Han T, Xie W, Li Y, Ma H, et al. Tolvaptan treatment improves survival of cirrhotic patients with ascites and hyponatremia. BMC Gastroenterol 2018;18:137.




93. Tang J, Wang Y, Han T, Mao Q, Cheng J, Ding H, et al. Tolvaptan therapy of Chinese cirrhotic patients with ascites after insufficient diuretic routine medication responses: a phase III clinical trial. BMC Gastroenterol 2020;20:391.




94. Dahl E, Gluud LL, Kimer N, Krag A. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther 2012;36:619-626.


95. Yan L, Xie F, Lu J, Ni Q, Shi C, Tang C, et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol 2015;15:65.




96. Li M, Bi Z, Huang Z. Impact of vaptans on clinical outcomes in cirrhosis patients: a meta-analysis of randomized controlled trials. Front Pharmacol 2019;10:1365.



97. Hayashi M, Abe K, Fujita M, Okai K, Takahashi A, Ohira H. Association between the serum sodium levels and the response to tolvaptan in liver cirrhosis patients with ascites and hyponatremia. Intern Med 2018;57:2451-2458.



98. Huh H, Kim YS, Chung W, Kim YL, Kim Y, Han S, et al. Evaluating the Safety and effectivenesS in adult KorEaN patients treated with Tolvaptan for management of autosomal domInAnt poLycystic kidney disease (ESSENTIAL): short-term outcomes during the titration period. Kidney Res Clin Pract 2023;42:216-228.




99. U.S. Food & Drug Administration (FDA). FDA drug safety communication: FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. FDA web site, <https://www.fda.gov/drugs/drugsafety-and-availability/fda-drug-safety-communication-fdalimits-duration-and-usage-samsca-tolvaptan-due-possibleliver>. Accessed 3 Mar 2023.
100. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al.; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012;367:2407-2418.



101. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-460 Erratum in: J Hepatol 2018;69:1207.


102. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: ascites and related complications. Clin Mol Hepatol 2018;24:230-277.




103. Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol 2016;51:629-650.



104. Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol 2013;11:123-130.e1.


105. Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-delArbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403-409.


106. Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al.; CONFIRM Study Investigators. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med 2021;384:818-828.

107. Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al.; ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an openlabel randomised trial. Lancet 2018;391:2417-2429. Lancet 2018;391:2417-2429 Erratum in: Lancet 2018;392:386.

108. Bai Z, Bernardi M, Yoshida EM, Li H, Guo X, M├®ndez-S├Īnchez N, et al. Albumin infusion may decrease the incidence and severity of overt hepatic encephalopathy in liver cirrhosis. Aging (Albany NY) 2019;11:8502-8525.



110. McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990;31:204-207.



111. Caraceni P, Angeli P, Prati D, Bernardi M, Berti P, Bennardello F, et al.; Italian Association for the Study of the Liver (AISF); on behalf of the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI). AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus 2021;19:9-13.


112. Shen NT, Barraza LH, Anam AK, Patel P, Schneider Y, Jesudian A. Benefit of albumin infusion in hospitalized patients with cirrhosis and hyponatremia: a retrospective cohort study. J Gastroenterol Hepatol Res 2017;6:2441-2445.

113. Bajaj JS, Tandon P, O╩╝Leary JG, Biggins SW, Wong F, Kamath PS, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol 2018;113:1339.



114. China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al. Targeted albumin therapy does not improve short-term outcome in hyponatremic patients hospitalized with complications of cirrhosis: data from the ATTIRE trial. Am J Gastroenterol 2021;116:2292-2295.


115. Zaccherini G, Baldassarre M, Tufoni M, Nardelli S, Piano S, Alessandria C, et al.; ANSWER Study Investigators. Correction and prevention of hyponatremia in patients with cirrhosis and ascites: post hoc analysis of the ANSWER study database. Am J Gastroenterol 2023;118:168-173.


116. Bai Z, Wang L, Lin H, Tacke F, Cheng G, Qi X. Use of human albumin administration for the prevention and treatment of hyponatremia in patients with liver cirrhosis: a systematic review and meta-analysis. J Clin Med 2022;11:5928.



117. Bai Z, Xu W, Chai L, Zheng X, M├®ndez-S├Īnchez N, Philips CA, et al. Effects of short-term human albumin infusion for the prevention and treatment of hyponatremia in patients with liver cirrhosis. J Clin Med 2022;12:107.



118. Chinese Society of Hepatology; Chinese Medical Association, Xu X, Duan Z, Ding H, Li W, Jia J, Wei L, et al. Chinese guidelines on the management of ascites and its related complications in cirrhosis. Hepatol Int 2019;13:1-21.



119. Hackworth WA, Heuman DM, Sanyal AJ, Fisher RA, Sterling RK, Luketic VA, et al. Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int 2009;29:1071-1077.


120. Kim JM, Kim DG, Kim J, Lee K, Lee KW, Ryu JH, et al. Outcomes after liver transplantation in Korea: incidence and risk factors from Korean transplantation registry. Clin Mol Hepatol 2021;27:451-462.




121. Cywinski JB, Mascha E, Miller C, Eghtesad B, Nakagawa S, Vincent JP, et al. Association between donor-recipient serum sodium differences and orthotopic liver transplant graft function. Liver Transpl 2008;14:59-65.


122. Romanovsky A, Azevedo LC, Meeberg G, Zibdawi R, Bigam D, Bagshaw SM. Serum sodium shift in hyponatremic patients undergoing liver transplantation: a retrospective cohort study. Ren Fail 2015;37:37-44.


123. Leise M, C├Īrdenas A. Hyponatremia in cirrhosis: implications for liver transplantation. Liver Transpl 2018;24:1612-1621.



124. Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014;21:1443-1450.


125. Lee EM, Kang JK, Yun SC, Kim KH, Kim SJ, Hwang KS, et al. Risk factors for central pontine and extrapontine myelinolysis following orthotopic liver transplantation. Eur Neurol 2009;62:362-368.



126. Morard I, Gasche Y, Kneteman M, Toso C, Mentha A, Meeberg G, et al. Identifying risk factors for central pontine and extrapontine myelinolysis after liver transplantation: a case-control study. Neurocrit Care 2014;20:287-295.



127. Alukal JJ, John S, Thuluvath PJ. Hyponatremia in cirrhosis: an update. Am J Gastroenterol 2020;115:1775-1785.


-
METRICS

- ORCID iDs
-
Seon Ha Baek

https://orcid.org/0000-0002-4751-9817Sejoong Kim

https://orcid.org/0000-0002-7238-9962 - Related articles
-
Letter regarding ŌĆ£Evidence-based hyponatremia management in liver diseaseŌĆØ2023 October;29(4)
Epidemiology and updated management for autoimmune liver disease2023 January;29(1)
The use of vaptan in hyponatremic patients with liver cirrhosis2011 December;17(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



