| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
ABSTRACT
Hepatitis B virus (HBV) infection was highly endemic in China, where the prevalence of HBsAg was 9.7% in 1992. Comprehensive strategies, including universal infant hepatitis B vaccination with emphasis on timely birth-dose and 3-dose coverage, dramatically reduced the mother-to-infant transmission and early childhood acquisition of HBV, resulting in estimated HBsAg prevalence rates of 5.6% and 0.1% in the general population and among children aged <5 years in 2022, respectively. Clinical guidelines on the prevention and treatment of chronic hepatitis B have been periodically updated based on emerging evidence from clinical research. The continuously improved reimbursement policy and the massively reduced price of antiviral drugs through government negotiation and central procurement have increased treatment accessibility and affordability. However, due to the low rates of diagnosis and treatment, China still faces a large challenge in achieving the 2030 goal of lowering HBV-related mortality by 65%. A public health approach involving concerted efforts from the government, medical community, industry, and society as a whole would be necessary to increase the uptake of HBV tests and treatment to achieve the global goal of eliminating viral hepatitis as a public health threat by 2030.
Chronic hepatitis B (CHB) poses a tremendous public health burden worldwide [1]. Progression to cirrhosis or hepatocellular carcinoma (HCC) occurs in approximately 15ŌĆō40% of untreated people with chronic infection of hepatitis B virus (HBV) [2]. The World Health Organization (WHO) estimated that the global seroprevalence of HBsAg was 3.8%, with 296 million people being chronically infected with HBV and nearly 820,000 people dying due to HBV-related disease in 2019 [3].
China bears a heavy CHB burden and has one-third of the worldŌĆÖs infected population [4,5]. To combat this infectious disease, China initiated universal infant hepatitis B vaccination (HepB) in 1992. With the increasing coverage rates of the full 3-dose regimen and a timely birth dose (TBD) of HepB, the prevalence of HBsAg has steadily declined in recent decades. However, the HBV-related disease burden is still high due to the enormous population and large number of people who have already been chronically infected with HBV. In this review, we describe the comprehensive approach to the control of CHB in China.
Since mother-to-child transmission (MTCT) and early childhood HBV infection play major roles in the high prevalence of hepatitis B surface antigen (HBsAg), China initiated a universal infant HepB immunization program in 1992, which recommends TBD within 24 hours of birth and second and third doses at one month and six months after birth, respectively (Fig. 1) [6,7].
In 2002, China integrated HepB vaccination into the Expanded Program on Immunization (EPI) and started to offer free HepB vaccines to all newborns, with the family paying only a small amount of the injection fee. In 2005, the government began to provide completely free HepB vaccination services to all newborns. The China Global Alliance for Vaccines and Immunization Project was conducted from 2002 to 2009 and delivered HepB to approximately 68 million children in hard-to-reach populations [7,8].
In addition, special projects were conducted in the resource-constrained western provinces from 2005 to 2009; these projects provided funding for training health care workers and subsidies for incentivizing baby delivery in facilities. These efforts increased the facility delivery rate (in township or county-level hospitals) from 43% to 97%, thereby increasing the rate of TBD of HepB from 82% to 88% in the resource-constrained western provinces [9].
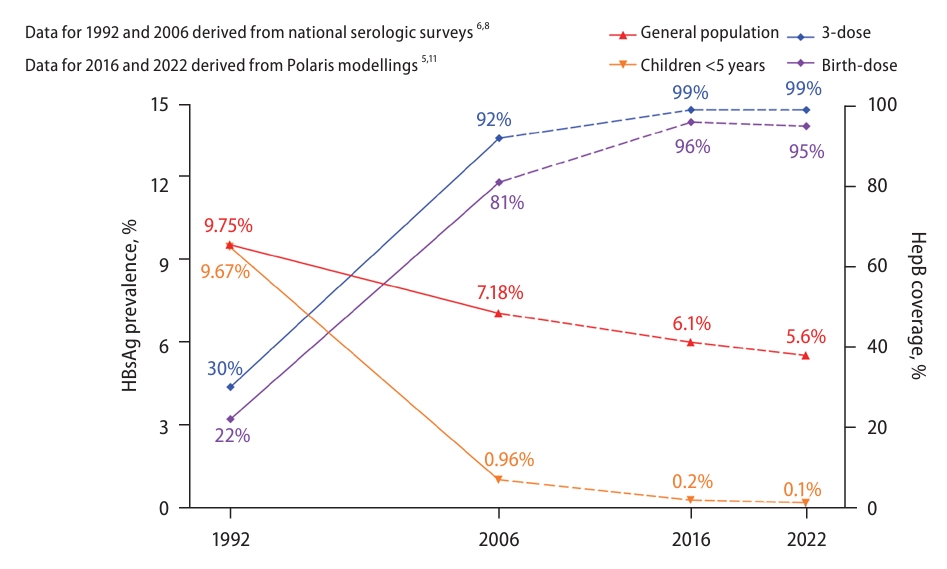
Owing to these efforts, three-dose HepB coverage tripled from 30.0% in 1992 to 99.6% in 2015, and the TBD rate increased from 22.2% to 95.6% during the same period [10]. As the coverage of HepB has steadily improved, disparities in the rates of 3-dose coverage and TBD coverage across regions and urban/rural areas were also eliminated by 2014 [6]. As a result, the prevalence rate of HBsAg among children under five years dramatically declined from 9.67% in 1992 to only 0.32% in 2014. 6 The results of the new nationwide seroprevalence survey conducted in 2020 have not been officially published. However, a modeling study estimated that the HBsAg prevalence in China was 5.6% in the general population and 0.1% among children <5 years of age in 2022 (Fig. 2) [5,6,8,11].
There is still a large population of HBsAg-positive women of child-bearing age in China. Indeed, it was reported that there were approximately one million infants were born to HBsAg-positive mothers in 2015 [12]. Consequently, a nonnegligible proportion of infants still acquire HBV infection from HBsAg-positive mothers, despite receiving a TBD and three doses of HepB. To further reduce the MTCT rate of HBV, a birth dose of hepatitis┬ĀB┬Āimmunoglobulin (HBIG) is administered to infants born to HBsAg-positive mothers [13]. In 2011, China launched a program of Triple Elimination of MTCT for human immunodeficiency virus (HIV), syphilis, and HBV, which provides complementary screening for HBsAg to all pregnant women and free HBIG to neonates born to HBsAg-positive mothers (Fig. 1) [14,15].
Finally, starting oral antiviral therapy in the second or third trimester of pregnancy in women with high HBV DNA levels could reduce the MTCT of HBV, as demonstrated by observational studies and a randomized controlled trial [16]. Later, this approach was recommended by the WHO guidelines for the prevention of MTCT of HBV. A real-world study (Shield Project Stage 1) showed that with comprehensive management, including passive and active immunoprophylaxis of infants and antiviral prophylaxis of mothers, the overall rate of HBV MTCT decreased to 0.9% [17].
To standardize the clinical management of preventing MTCT of HBV and further reduce HBV infection among infants, the China Foundation for Hepatitis Prevention and Control published an expert-recommended algorithm for the prevention of MTCT of HBV in 2022 [13]. This updated algorithm suggests tenofovir disoproxil fumarate (TDF) administration to pregnant women starting at 28 weeks of gestation after informed consent; tenofovir alafenamide fumarate (TAF) could also be used for pregnant women who have or are at high risk for osteoporosis or renal function issues [13].
In China, horizontal transmission also plays a role in HBV infection, which is acquired later in life, although it is estimated that up to half of chronic HBV infections occur via MTCT or horizontal transmission in early childhood [18]. The elucidation of factors associated with chronic HBV infection may help inform targeted screening in the general population. Indeed, a meta-analysis demonstrated that a set of factors, including middle age, male sex, being married, rural residence, lower educational level, smoking, having an HBsAg-positive household contact or family history of HBV, and history of surgery or blood transfusion, was significantly associated with higher HBsAg prevalence [19]. Since 1998, China has banned paid blood donors and has conducted stringent screenings of volunteer blood donors, with all donated blood being tested for HBV DNA since 2015 [6].
Injection safety has also improved in the last two decades. The government issued a rule banning the reuse of nonsterilizable medical devices in 2000, and the Chinese Medical Association published clinical guidelines for injections and other skin-piercing procedures in 2005. In 2007, auto-disposable syringes became available for vaccine injections. By 2010, all reusable injection equipment was eliminated in China [20,21].
Harm reduction interventions for people who inject drugs (PWID) have also been integrated into comprehensive HBV prevention strategies in China. A systematic review of studies published from 2008ŌĆō2017 revealed that the pooled HBsAg prevalence was 19.6% (95% confidence interval 13.7ŌłÆ25.5%) for PWID in China, with the highest occurring in South China (25.3%, 14.6ŌłÆ36.0%), followed by Central China (20.8%, 17.4ŌłÆ24.1%), and the lowest occurring in North China (15.9%, 12.8ŌĆō19.4%) (Table 1) [22]. A study showed that the annual number of needles and syringes distributed was estimated to be 208 per PWID, and the rate of safe injection use among PWID was 86.5% in 2015 [23].
In the past two decades, there has been steady growth in hepatology research and publications from China [24]. The continuous support of the National Natural Science Foundation of China (NSFC) has been instrumental to such progress. From 1986 to 2017, the NSFC provided a total of 3.67 billion RMB (Chinese Yuan) to 8,587 liver research projects, with HBV projects accounting for 12.2% of the total number of projects (1,044/8,587) [24]. In addition, since 2008, major research programs on HIV, viral hepatitis, and tuberculosis have been initiated in China, which has supported hundreds of research projects that yielded high-quality clinical evidence on HBV prevention and treatment [25-32].
The guidelines on the prevention and treatment of CHB were first jointly published by the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases in 2005 and updated in 2010, 2015, 2019, and 2022 (Fig. 3) [33]. Nationwide educational activities have been conducted to promote the implementation of the HBV guidelines, especially in rural and resource-constrained areas [34].
The newly updated CHB guidelines recommend treating all patients with viremia, together with elevated alanine aminotransferase levels, or any evidence of cirrhosis/advanced liver fibrosis (by invasive or noninvasive measurements), age Ōēź30 years, or family history of HBV-related cirrhosis or HCC [33]. The current Chinese guidelines recommend first-line nucleot(s) ide analogs (NAs), including entecavir (ETV), TDF, TAF, tenofovir amibufenamide and pegylated interferon alpha (PEG-IFN╬▒), for patients with CHB [33]. PEG-IFN-╬▒ is now classified as Category B (partially reimbursable) in the reimbursement list of basic social medical insurance in China, yet the exact proportion of reimbursement differs across cities/provinces, depending on their local policies, which are mainly determined by economic status.
Notably, over the last two decades, the application of PEG-IFN-╬▒ for CHB treatment in China has evolved considerably. Currently, the notion of highly selected patients with favorable baseline predictors (HBV DNA suppression, HBeAg seroconversion, and very low levels of HBsAg) is widely recognized in the hepatology community [35-37]. PEG-IFN-╬▒ is indicated for treatment-naive patients or those already receiving NAs as monotherapy, add-on therapy, or de novo combination therapy. Treatment durations might extend beyond the standard of 48 weeks to 72, 96, or even 120 weeks in highly selected patients with favorable baseline (HBsAg <1,500 IU/mL) and on-treatment (HBsAg <200 IU/mL) features for a greater chance of HBsAg loss [38].
Current antiviral drugs can suppress HBV replication and reduce liver disease progression, thus improving the long-term outcomes of CHB patients [39,40]. However, the high price and limited reimbursement for HBV therapy used to be major hurdles to its wide clinical use [41].
To improve the accessibility of antiviral therapy for HBV, NAs and IFN-╬▒ (conventional and PEG-IFN-╬▒) have been included on the reimbursement list since 2010. By 2017, ETV, TDF, TAF and PEG-IFN-╬▒ were all included in the updated national list of reimbursable medicines in China [42]. The reimbursement criteria for antiviral therapy for CHB have evolved considerably in China. In general, the reimbursement criteria reflect the updated treatment guidelines, with only minor variation at the city or province level.
In 2019, the General Office of the State Council of the PeopleŌĆÖs Republic of China issued the National Centralized Drug Procurement (NCDP) policy to improve the accessibility and affordability of medicines [43]. This was the first attempt at nationwide volume-based drug (mainly generic) procurement in the mainland of China, aiming at providing high-quality drugs at lower prices. Based on the data from the National Healthcare Security Administration, before the implementation of the NCDP policy, the average per person-year prices of the original brand names of ETV, TDF, and TAF were 650 RMB (90.90 USD), 1139 RMB (159.30 USD) and 6480 RMB (906.29 USD), respectively. These figures dramatically declined to 70 RMB (9.79 USD), 180 RMB (25.14 USD) and 360 RMB (50.35 USD) per person-year for the corresponding generic drugs after the adoption of the NCDP policy (per the PeopleŌĆÖs Bank of China, the exchange rate of RMB to USD was 7.15 to 1 on November 27, 2023). Moreover, the prices of brand name ETV, TDF, and TAF have also dramatically decreased [44]. As a result, the NCDP policy in China has substantially reduced drug costs, increasing the accessibility and affordability of antiviral treatment (Fig. 3) [44].
As shown by data analysis of the China Registry of Hepatitis B (CR-HepB), the proportion of ETV and TDF usage in those who received any antiviral agents had increased from 13.5% in 2003 to 79.7% in 2016 [45]. Basic social medical insurance coverage of first-line medicines for antiviral treatment was associated with a substantially reduced risk of liver-related death, from 0.38% to 0.16% for patients with noncirrhotic CHB and from 4.03% to 3.39% for those with compensated cirrhosis in Beijing, China [46].
One study showed that 98.22% of hepatitis-related deaths from liver disease in China were attributed to HBV-related cirrhosis/other chronic liver diseases (26.04%) and HBV-related liver cancer (72.18%) in 2019 [47]. Analysis of the Global Burden of Diseases (GBD) data showed that HBV accounted for 68% of cirrhosis cases and 65% of HCC cases in China [48,49]. The main indications for liver transplantation in China are HBV-related cirrhosis, with or without acute-on-chronic liver failure (ACLF) and HCC. HBV infection was the most common cause of ACLF (66.24%), followed by alcoholic liver disease (10.56%), autoimmune liver disease (6.53%) and hepatitis C virus infection (2.25%) [50]. Although a favorable trend in the mortality of liver disease due to hepatitis B was observed between 1990 and 2019 [51]. China still faces challenges in achieving the WHOŌĆÖs goal by 2030 [52].
The prevalence and magnitude of reduction in chronic HBV infection vary by geographical region and across other strata of the Chinese population. In the mainland of China, the highest prevalence in the latest study period was observed in the Xizang Autonomous Administrative Region (formerly known as Tibet), followed by other provinces mostly clustered in southern China (Hainan, Jiangxi, Fujian, Guangxi, Guangdong and Qinghai), whereas the lowest seroprevalence was observed in Beijing and Shanxi [53]. This geographical heterogeneity in HBsAg prevalence is consistent with that observed in a recently published study among nearly 100 million pregnant women across 31 provinces in China [54]. The various HBV infection rates could be explained by several factors, but the major factor may be the difference in the rates of 3-dose coverage and TBD coverage of HepB after the implementation of the universal infant HepB program. In 1999, a national EPI review showed that in Beijing, the coverage rates of TBD and three-dose HepB among 1-year-old children were 69.0% and 99.0%, respectively, whereas in Xizang, the coverage rates for 2-year-old children were only 7.8% and 2.1%, respectively [55]. As mentioned above, disparities in the 3-dose coverage rate and TBD coverage rate across regions and urban/rural areas were largely eliminated by 2014 [6].
In 2022, it is estimated that in China, there were still 79,747,000 people living with chronic HBV infection; however, only 19,131,000 (24%) were diagnosed, and 5,076,000 of 33,874,000 (15%) who were potentially eligible for treatment were actually receiving treatment [11]. Therefore, efforts should be focused on increasing the coverage of the diagnosis and treatment of hepatitis B in China.
A modeling study estimated that if we treat only the few millions of individuals with CHB who are already on treatment, HBV-related cirrhosis/HCC mortality will continue to increase over the next two decades; if we scale up test-and-treatment strategies and treat most or even all people who need treatment, HBV-associated mortality will dramatically decline, which is cost-effective or even cost-saving [56]. Another modeling study also demonstrated that China would spend 55 billion USD extra money and lose 334,000 more lives by a 1-year delay in achieving the 80% treatment goal by 2030 [57].
A modeling study by Su and colleagues showed that universal screening for HBV among adults aged 18ŌĆō70 years is cost-effective and could identify most infected individuals in China [58]. Another modeling study showed that treating all HBsAg-positive individuals aged 18ŌĆō80 years could facilitate the achievement of the 2030 target of a 65% reduction in HBV-related deaths and is cost-effective [59].
To increase the diagnostic rate of CHB and scale up diagnosis and treatment, the following measures can be taken. First, advocacy is necessary to increase awareness of the HBV-related disease burden and convey that antiviral therapy is cost effective [57]. Second, the treatment algorithm should be simplified with expanded antiviral indications as recommended in the updated guidelines [34]. Third, capacity building should be strengthened by conducting continuous medical education to train local doctors in remote or economically underprivileged areas to promote the new treatment guidelines. Fourth, an HBV-specific notification and reporting system should be established to monitor the diagnosis and treatment rates and facilitate evidence-based decision-making. Finally, pilot microelimination programs should be implemented at the township, countywide, or even city/province level to explore the feasibility of large-scale testing and treatment initiatives, including capacity building, task shifting, and link-to-care models, by exploiting instant communication and social media apps.
In summary, China has adopted a comprehensive strategy involving the motivational investment of various resources and achieved great success in HBV prevention and control. However, China still faces a large challenge in reducing HBV mortality through large-scale testing and treating the large number of people who are already infected with HBV. The concerted efforts of the government, medical community, industry, civil society, and the public are key to eliminating CHB as a public health threat by 2030 in China.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No. 20212YFC2303600) and the National Natural Science Foundation of China (No. 82000569 and 82270603).
FOOTNOTES
Figure┬Ā1.
Milestones for the prevention of HBV infection in China. HBV, hepatitis B virus; HIV, human immunodeficiency virus; MTCT, motherto-child transmission.

Figure┬Ā2.
Increasing HepB coverage and decreasing HBsAg prevalence in China. HepB, hepatitis B vaccination; HBsAg, hepatitis B surface antigen.
Figure┬Ā3.
Milestones for the treatment of CHB in China. CHB, chronic hepatitis B; NCDP, National Centralized Drug Procurement; HBV, hepatitis B virus.

Table┬Ā1.
Estimate of the prevalence of HBsAg among PWID in China by region (2008ŌĆō2017)
Abbreviations
HBV
hepatitis B virus
CHB
chronic hepatitis B
HCC
hepatocellular carcinoma
HepB
hepatitis B vaccination
TBD
timely birth dose
MTCT
mother-to-child transmission
EPI
Expanded Program on Immunization
HBIG
hepatitis B immunoglobulin
HIV
human immunodeficiency virus
TAF
tenofovir alafenamide fumarate
TDF
tenofovir disoproxil fumarate
AD
auto-disposable
PWID
people who inject drugs
ALT
alanine aminotransferase
NAs
nucleot(s)ide analogs
ETV
entecavir
TMF
tenofovir amibufenamide
PEG-IFN╬▒
pegylated interferon alpha
ACLF
acute-on-chronic liver failure
HCV
hepatitis C virus
REFERENCES
1. Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol 2022;22:19-32.



3. World Health Organization (WHO). Global progress report on HIV, viral hepatitis and sexually transmitted infections; 2021;WHO web site, <https://www.who.int/publications/i/item/9789240027077>. Accessed 31 Aug 2023.
4. Chen S, Li J, Wang D, Fung H, Wong LY, Zhao L. The hepatitis B epidemic in China should receive more attention. Lancet 2018;391:1572.


5. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383-403.

6. Cui F, Shen L, Li L, Wang H, Wang F, Bi S, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis 2017;23:765-772.



7. Kane MA, Hadler SC, Lee L, Shapiro CN, Cui F, Wang X, et al. The inception, achievements, and implications of the China GAVI Alliance Project on Hepatitis B Immunization. Vaccine 2013;31 Suppl 9:J15-20.


8. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550-6557.


9. Hutin Y, Hennessey K, Cairns L, Zhang Y, Li H, Zhao L, et al. Improving hepatitis B vaccine timely birth dose coverage: lessons from five demonstration projects in China, 2005-2009. Vaccine 2013;31 Suppl 9:J49-55.


10. Chen S, Mao W, Guo L, Zhang J, Tang S. Combating hepatitis B and C by 2030: achievements, gaps, and options for actions in China. BMJ Glob Health 2020;5:e002306.



11. Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol 2023;8:879-907.

12. Cui F, Woodring J, Chan P, Xu F. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China. Int J Epidemiol 2018;47:1529-1537.


13. Liu Z, Chen Z, Cui F, Ding Y, Gao Y, Han G, et al. Management algorithm for prevention of mother-to-child transmission of hepatitis B virus (2022). J Clin Transl Hepatol 2022;10:1004-1010.



14. Shan S, Jia J. Prevention of mother-to-child transmission of hepatitis B virus in the Western Pacific Region. Clin Liver Dis (Hoboken) 2021;18:18-21.




15. Wang AL, Qiao YP, Wang LH, Fang LW, Wang F, Jin X, et al. Integrated prevention of mother-to-child transmission for human immunodeficiency virus, syphilis and hepatitis B virus in China. Bull World Health Organ 2015;93:52-56.



16. Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016;374:2324-2334.


17. Yin X, Han G, Zhang H, Wang M, Zhang W, Gao Y, et al. A real-world prospective study of mother-to-child transmission of HBV in China using a mobile health application (Shield 01). J Clin Transl Hepatol 2020;8:1-8.



18. Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol 2011;21:401-416.



19. Hamilton EM, Rassam W, Yan Y, Singh A, Ng SYA, Zhang J, et al. Correlates of chronic hepatitis B virus infection in the general adult population of China: Systematic review and meta-analysis. J Viral Hepat 2023;30:470-488.



20. Wu Z, Cui F, Chen Y, Miao N, Gong X, Luo H, et al. Evaluation of immunization injection safety in China, 2010: achievements, future sustainability. Vaccine 2013;31 Suppl 9:J43-J48.

21. Shan S, Cui F, Jia J. How to control highly endemic hepatitis B in Asia. Liver Int 2018;38 Suppl 1:122-125.



22. Bao Y, Larney S, Peacock A, Colledge S, Grebely J, Hickman M, et al. Prevalence of HIV, HCV and HBV infection and sociodemographic characteristics of people who inject drugs in China: A systematic review and meta-analysis. Int J Drug Policy 2019;70:87-93.



23. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ 2019;97:230-238.



24. Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol 2019;71:212-221.


25. Zeng N, Zou C, He Z, Ma H, Ou X, You H, et al. Systematic review on the reporting quality of randomized controlled trials in patients with hepatitis B or C in China. Int J Infect Dis 2018;67:58-64.


26. Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao H, et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology 2017;65:1438-1450.



27. Kong Y, Sun Y, Zhou J, Wu X, Chen Y, Piao H, et al. Early steep decline of liver stiffness predicts histological reversal of fibrosis in chronic hepatitis B patients treated with entecavir. J Viral Hepat 2019;26:576-585.


28. Wu S, Kong Y, Piao H, Jiang W, Xie W, Chen Y, et al. On-treatment changes of liver stiffness at week 26 could predict 2-year clinical outcomes in HBV-related compensated cirrhosis. Liver Int 2018;38:1045-1054.

29. Wu X, Zhou J, Sun Y, Ding H, Chen G, Xie W, et al. Prediction of liver-related events in patients with compensated HBV-induced cirrhosis receiving antiviral therapy. Hepatol Int 2021;15:82-92.



30. Zhang W, Wang X, Wang Y, Zhao X, Duan W, Wang Q, et al. Effective viral suppression is necessary to reduce hepatocellular carcinoma development in cirrhotic patients with chronic hepatitis B: Results of a 10-year follow up. Medicine (Baltimore) 2017;96:e8454.


31. Wang Q, Zhao H, Deng Y, Zheng H, Xiang H, Nan Y, et al. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis. J Hepatol 2022;77:1564-1572.


32. Wu X, Hong J, Zhou J, Sun Y, Li L, Xie W, et al. Health-related quality of life improves after entecavir treatment in patients with compensated HBV cirrhosis. Hepatol Int 2021;15:1318-1327.



33. You H, Wang F, Li T, Xu X, Sun Y, Nan Y, et al. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). J Clin Transl Hepatol 2023;11:1425-1442.



34. Wang Y, Wang M, Zhang G, Ou X, Ma H, You H, et al. Control of chronic hepatitis B in China: Perspective of diagnosis and treatment. China CDC Wkly 2020;2:596-600.



35. Hu C, Song Y, Tang C, Li M, Liu J, Liu J, et al. Effect of pegylated interferon plus tenofovir combination on higher hepatitis B surface antigen loss in treatment-naive patients with hepatitis B e Antigen -positive chronic hepatitis B: A real-world experience. Clin Ther 2021;43:572-581.e3.


36. Hu P, Shang J, Zhang W, Gong G, Li Y, Chen X, et al. HBsAg Loss with Peg-interferon Alfa-2a in Hepatitis B Patients with Partial Response to Nucleos(t)ide Analog: New Switch Study. J Clin Transl Hepatol 2018;6:25-34.



37. Chu JH, Huang Y, Xie DY, Deng H, Wei J, Guan YJ, et al. Real-world study on HBsAg loss of combination therapy in HBeAg-negative chronic hepatitis B patients. J Viral Hepat 2022;29:765-776.

38. Li M, Zhang L, Lu Y, Chen Q, Lu H, Sun F, et al. Early serum HBsAg kinetics as predictor of HBsAg loss in patients with HBeAg-Negative chronic hepatitis B after treatment with pegylated interferon╬▒-2a. Virol Sin 2021;36:311-320.




39. Jia J, Tang H, Ning Q, Jiang J, Dou X, Zhang M, et al. Real-world evidence for nucleoside/nucleotide analogues in a 5-year multicentre study of antiviral-naive chronic hepatitis B patients in China: 52-week results. Antivir Ther 2018;23:201-209.



40. Hou JL, Zhao W, Lee C, Hann HW, Peng CY, Tanwandee T, et al. Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries. Clin Gastroenterol Hepatol 2020;18:457-467.e21.


41. Qiu Q, Duan XW, Li Y, Yang LK, Chen Y, Li H, et al. Impact of partial reimbursement on hepatitis B antiviral utilization and adherence. World J Gastroenterol 2015;21:9588-9597.



42. Li M, Zhao L, Zhou J, Sun Y, Wu X, Ou X, et al. Changing clinical care cascade of patients with chronic hepatitis B in Beijing, China. Lancet Reg Health West Pac 2021;16:100249.



43. The State Council the PeopleŌĆÖs Republic of China. The Notice of the General Office of the State Council on Issuing National Drug Centralized Purchasing and Using Pilot Program (2019) [EB/OL]. GOV web site, <https://www.gov.cn/zhengce/content/2019-01/17/content_5358604.htm>. Accessed 31 Aug 2023.
44. Zhao X, Li M, Wang H, Xu X, Wu X, Sun Y, et al. Impact of national centralized drug procurement policy on antiviral utilization and expenditure for hepatitis B in China. J Clin Transl Hepatol 2022;10:420-428.



45. Shan S, You H, Niu J, Shang J, Xie W, Zhang Y, et al. Baseline characteristics and treatment patterns of the patients recruited to the China registry of hepatitis B. J Clin Transl Hepatol 2019;7:322-328.


46. Li M, Kong YY, Wu SS, Zhou JL, Wu XN, Wang L, et al. Impact of reimbursement program on liver-related mortality in patients with chronic hepatitis B in Beijing, China. J Dig Dis 2019;20:467-475.



47. Cao G, Liu J, Liu M. Trends in mortality of liver disease due to hepatitis B in China from 1990 to 2019: findings from the Global Burden of Disease Study. Chin Med J (Engl) 2022;135:2049-2055.



48. Alberts CJ, Clifford GM, Georges D, Negro F, Lesi OA, Hutin YJ, et al. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol Hepatol 2022;7:724-735.



49. GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022;7:796-829.


50. Ling S, Jiang G, Que Q, Xu S, Chen J, Xu X. Liver transplantation in patients with liver failure: Twenty years of experience from China. Liver Int 2022;42:2110-2116.



51. Yue T, Zhang Q, Cai T, Xu M, Zhu H, Pourkarim MR, et al. Trends in the disease burden of HBV and HCV infection in China from 1990-2019. Int J Infect Dis 2022;122:476-485.


52. Cao G, Jing W, Liu J, Liu M. Countdown on hepatitis B elimination by 2030: the global burden of liver disease related to hepatitis B and association with socioeconomic status. Hepatol Int 2022;16:1282-1296.



53. Liu Z, Lin C, Mao X, Guo C, Suo C, Zhu D, et al. Changing prevalence of chronic hepatitis B virus infection in China between 1973 and 2021: a systematic literature review and meta-analysis of 3740 studies and 231 million people. Gut 2023;72:2354-2363.



54. Liu J, Wang X, Wang Q, Qiao Y, Jin X, Li Z, et al. Hepatitis B virus infection among 90 million pregnant women in 2853 Chinese counties, 2015-2020: a national observational study. Lancet Reg Health West Pac 2021;16:100267.



55. Zhu X, Zhang X, Wang L. National EPI vaccination and hepatitis B vaccine coverage rate and the related factors: results from the 1999 nationwide survey. Chinese J Vacc Immun 2000;6:193-197.
56. Nayagam S, Chan P, Zhao K, Sicuri E, Wang X, Jia J, et al. Investment case for a comprehensive package of interventions against hepatitis B in China: Applied modeling to help national strategy planning. Clin Infect Dis 2021;72:743-752.




57. Toy M, Hutton D, Jia J, So S. Costs and health impact of delayed implementation of a national hepatitis B treatment program in China. J Glob Health 2022;12:04043.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,432 View
- 125 Download
- ORCID iDs
-
Jidong Jia

https://orcid.org/0000-0002-4673-8890 - Related articles
-
The imitator of immune-tolerant chronic hepatitis B: A killer in disguise2023 April;29(2)
RNA interference as a novel treatment strategy for chronic hepatitis B infection2022 July;28(3)
Cost-effectiveness of chronic hepatitis C screening and treatment2022 April;28(2)
Unmet need in chronic hepatitis B management2019 June;25(2)
New perspectives of biomarkers for the management of chronic hepatitis B2016 December;22(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



