| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
See the commentary-article "Steatotic liver disease: Know your enemies" on page 171.
ABSTRACT
Background/Aims
Nonalcoholic fatty liver disease (NAFLD) is associated with a multitude of adverse outcomes. We aimed to estimate the pooled incidence of NAFLD-related adverse events.
Methods
We performed a systematic review and meta-analysis of cohort studies of adults with NAFLD to evaluate the pooled incidence of adverse events.
Results
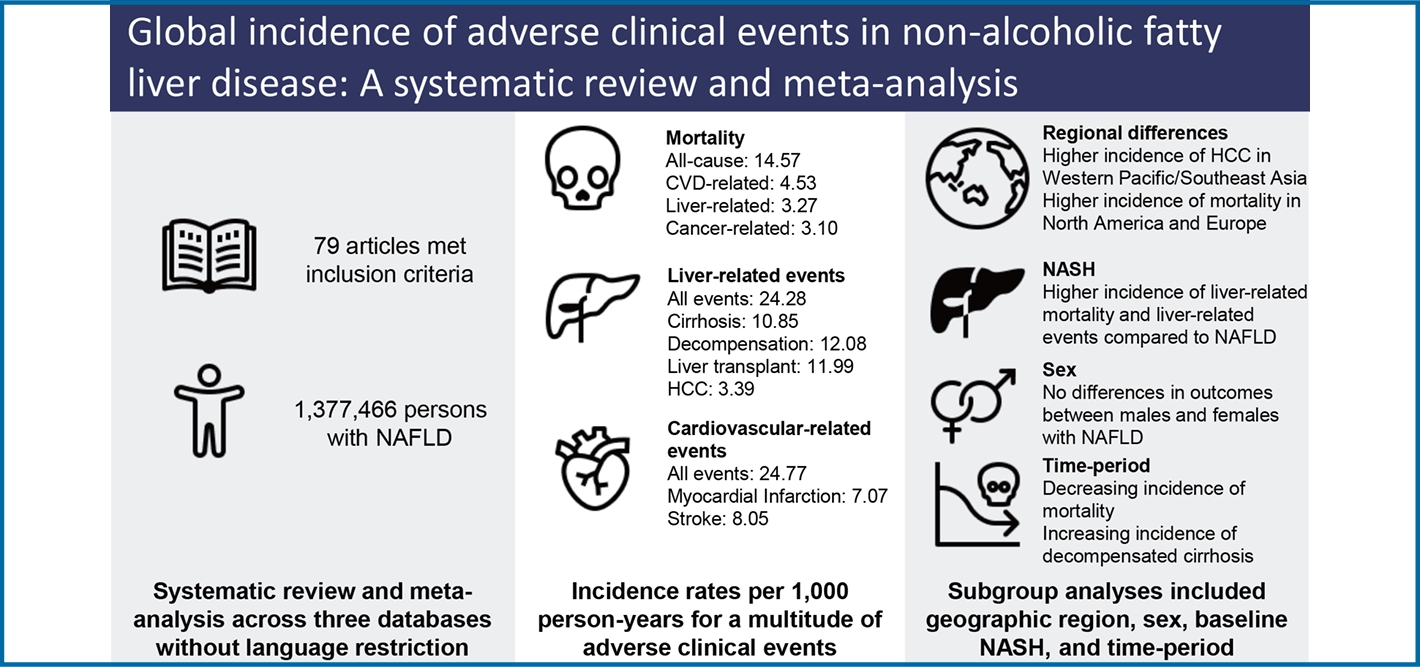
19,406 articles were screened, 409 full-text articles reviewed, and 79 eligible studies (1,377,466 persons) were included. Mean age was 51.47 years and body mass index 28.90 kg/m2. Baseline comorbidities included metabolic syndrome (41.73%), cardiovascular disease (CVD) (16.83%), cirrhosis (21.97%), and nonalcoholic steatohepatitis (NASH) (58.85%). Incidence rate per 1,000 person-years for mortality included: all-cause (14.6), CVD-related (4.53), non-liver cancer-related (4.53), and liver-related (3.10). Incidence for liver-related events included overall (24.3), fibrosis progression (49.0), cirrhosis (10.9), liver transplant (12.0), and hepatocellular carcinoma (HCC) (3.39). Incidence for non-liver events included metabolic syndrome (25.4), hypertension (25.8), dyslipidemia (26.4), diabetes (19.0), CVD (24.77), renal impairment (30.3), depression/anxiety (29.1), and non-liver cancer (10.5). Biopsy-proven NASH had higher incidence of HCC (P=0.043) compared to non-NASH. Higher rates of CVD and mortality were observed in North America and Europe, hypertension and non-liver cancer in North America, and HCC in Western Pacific/Southeast Asia (P<0.05). No significant differences were observed by sex. Time-period analyses showed decreasing rates of cardiovascular and non-liver cancer mortality and increasing rates of decompensated cirrhosis (P<0.05).
Graphical Abstract
Nonalcoholic fatty liver disease (NAFLD), a complex chronic liver disease associated with metabolic disorders in particular obesity and type 2 diabetes mellitus, is a major global health problem affecting more than 30% of the global population as of 2019 [1-3]. NAFLD carries a significant clinical burden including increased risk for hepatocellular carcinoma (HCC), non-liver cancer, cardiovascular disease (CVD), as well as increased risk for all-cause, liver-related, and CVD-related mortality [4-6].
With progress in the diagnosis and treatment of viral hepatitis, NAFLD is poised to become the leading cause of liver-related morbidity and mortality in the world [7]. NAFLD is already the leading etiology for cirrhosis in Mexico and is now the fastest growing indication for liver transplantation and HCC in liver transplant candidates in the United States [8-10]. Similar trends for NAFLD-related HCC have been noted in Europe [11,12].
NAFLD also carries a significant economic burden with annual direct medical costs estimated at $101 billion in the United States, Ōé¼35 billion in Europe, and even higher in those with diabetes mellitus (DM) [13,14]. As the global prevalence of diabetes and obesity continue to increase, the prevalence of NAFLD is forecasted to affect approximately 50% of the global population by 2040 [15]. In fact, the World Obesity Foundation recently released a report stating that without effective strategies to change the trajectory, over 50% of the world population will be overweight or obese by 2035 with an economic cost greater than $4 trillion, suggesting that the costs for NAFLD will continue to escalate as well [16]. From the patientŌĆÖs perspective, those with NAFLD reported fatigue, depression, lack of ability to physically perform their activities of daily living, and reduced work productivity [17].
In June 2023, a multi-society consensus was made to change the nomenclature from NAFLD to Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) with new diagnostic criteria focusing on cardiometabolic risk factors [18]. Given the newly proposed diagnostic criteria and nomenclature change, concerns have been raised regarding the applicability of NAFLD research to this new definition. A study of patients from Hong Kong found 6/261 (2.3%) MR spectroscopy-diagnosed and 1/414 (0.2%) biopsy-proven NAFLD were unable to be classified as MASLD [19]. Additionally, a population-based study from the United States utilizing data from the National Health and Nutrition Examination Survey found a 99% overlap between NAFLD and MASLD [20]. Given the minimal discrepancy between MASLD and NAFLD, the findings from NAFLD studies will likely remain applicable under this new nomenclature.
Despite our knowledge of NAFLD prevalence, incidence, and its outcomes, incidence rate data for associated adverse outcomes is still sparse [21]. Therefore, to provide more targeted interventions for people with NAFLD, it is vital to understand the incidence of adverse clinical outcomes in this population. This study aimed to fill this knowledge gap by identifying the incidence of the major health-related events occurring in people with NAFLD by systematic review and meta-analysis.
We performed a systematic review and meta-analysis to evaluate the outcomes of NAFLD according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for the conduct of meta-analyses of observational studies (http://www.prisma-statement.org/; Supplementary Table 1) [22].
Our search strategy was developed in collaboration with a medical librarian (CDS, Stanford Lane Library). The search was conducted in three databases without language restriction (PubMed, EMBASE, and Cochrane Library from inception to June 22, 2021) using keywords such as ŌĆ£NAFLDŌĆØ and ŌĆ£epidemiology.ŌĆØ Additional details can be found in the Supplementary File. Two authors independently performed the literature search and selected relevant articles. Discrepancies were resolved via consensus and/or discussion with a third author. Observational cohort studies of adult persons aged 18 years or older who had NAFLD at baseline, described the number of persons who reached an outcome of interest, and provided follow-up time data included. We excluded studies that included persons under the age of 18, special populations (e.g., viral hepatitis coinfection, hemodialysis patients), or studies that focused exclusively on a patient subgroup (e.g., elderly patients). Non-observational studies were excluded. For studies with overlapping patient populations, articles that provided the most data (largest patient sample, most subgroup data, most updated data) were selected.
Patients were considered to have NAFLD if they had evidence of hepatic steatosis in the absence of heavy alcohol use and other underlying liver diseases. Data on diagnostic method for NAFLD were collected (ultrasound, biopsy, noninvasive indices, and NAFLD diagnosis codes). Data were collected on several outcomes including mortality (all-cause, CVD-related, liver-related, and non-liver cancer-related), liver-related outcomes (fibrosis progression, cirrhosis, liver transplant, and HCC), decompensation (ascites, varices/variceal bleeding, hepatic encephalopathy), metabolic-related events (metabolic syndrome [MetS], hypertension [HTN], hyperlipidemia/dyslipidemia [HLD/DLD], DM), cardiovascular events (coronary artery disease/congestive heart failure [CAD/CHF], myocardial infarction [MI], ischemic/hemorrhagic stroke), renal impairment, depression/anxiety, and non-liver cancer. Additional data were collected on demographic, geographic region, and presence of nonalcoholic steatohepatitis (NASH) at baseline. Study time was described via calculation of median study time from the recorded study initiation and end dates.
The primary endpoint was the incidence of adverse events in persons with NAFLD. Numerators were the number of persons with NAFLD at the start of the study. Denominators were the person-years of follow-up during the study period, which was determined via direct extraction of person-years follow-up time by the original studies, or by multiplying the total number of persons at risk with the mean/median year of study follow-up. We extracted the total number of persons with NAFLD at baseline within each cohort and the total number of persons who attained each outcome during the study period.
A specific case report form was developed for which two authors independently extracted data from eligible studies. Discrepancies in data extraction were resolved via consensus or consultation with a third author. A modified Newcastle-Ottawa scale with 5 main criteria and a maximum of 9 points was utilized for quality assessment (Supplementary Table 2). Studies with total scores of 7ŌĆō9 were considered to have good quality, 4ŌĆō6 as fair, and <4 as poor.
Random-effects models were used to estimate the pooled incidence of adverse events among NAFLD patients. The Cochran Q-statistic and I2 statistic was used to assess heterogeneity with estimates associated with P-value of <0.05 in Q-statistic and I2 Ōēź50% considered as having significant heterogeneity. The effects of heterogeneity on the incidence of adverse events were investigated via pre-planned subgroup analyses that included geographic region, sex, timeperiod, and presence of NASH at baseline, as per available data from individual studies. Standard EggerŌĆÖs test, DerSimonian-Lair and Sidik-Jonkman random-effects model adjustments, and funnel plot were used to assess for publication bias. P-values were generated using the standard DerSimonian and Laird random-effects models. Statistical analyses were conducted using the meta suite of commands in R statistical software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
Our search strategy identified 19,406 articles from PubMed, Embase, and Cochrane Library. After removing 4,401 duplicates, 15,005 citations were left for abstract and title screening, and 502 articles were subsequently identified for fulltext screening. Finally, 79 articles met inclusion/exclusion criteria and were included in analysis. Among the studies that met inclusion criteria, 31 used ultrasound for diagnosis, 29 biopsy, 13 via International Classification of Diseases 10 (ICD-10) codes, and 6 other imaging (magnetic resonance imaging, computerized tomography, mixed imaging) (Fig. 1). Outcomes reported included mortality (n=37), liver-related events (n=40), non-liver cancer (n=11), decompensated cirrhosis (n=16), MetS (n=3), HTN (n=12), HLD/DLD (n=7), DM (n=24), cardiovascular events (n=19), renal events (n=6), and depression/anxiety (n=1). Median study year spanned from 1987 to 2017 (median: 2007) and study sample size ranged from 52 to 262,619 persons. Median study follow-up time was 6.46 years or 5,314.5 person-years. Additional characteristics can be found in Supplementary Table 2.
Using modified Newcastle-Ottawa scale, the majority (74%) of studies were good quality with a median score of 8 with the remaining being fair quality (26%) with a median score of 6 (Supplementary Table 3). EggerŌĆÖs test noted no statistically significant publication biases in each outcome (Supplementary Fig. 1AŌĆōH).
Table 1 displays the pooled baseline characteristics of patients with NAFLD. The mean age was 51.47 years, mean body mass index was 28.90 kg/m2, 28.66% had DM, 41.73% had MetS, 16.83% had CVD, 21.97% had cirrhosis and 58.85% had NASH.
Pooled incidence rates per 1,000 person-years were 14.57 for all-cause, 4.45 for CVD-related, 3.27 for non-liver cancer related, and 3.10 for liver-related mortality (Table 2).
Pooled incidence rates of liver-related events per 1,000 person-years were 24.28 overall, 3.39 for HCC, 10.85 for cirrhosis, 12.08 for hepatic decompensation (varices/variceal hemorrhage [4.72], ascites [6.68], hepatic encephalopathy [2.31]), and 11.99 for liver transplant (Table 2).
Pooled incidence rates for metabolic events were: MetS (25.40), HTN (25.84), HLD/DLD (26.43), and DM (19.01) (Table 2).
Pooled incidence rates for cardiovascular events were 24.77 overall (40.12 CAD/CHF, 7.07 for MI). Pooled incidence rates for other non-liver events were as follows: 30.28 for renal events, 29.10 for depression/anxiety, and 10.48 for non-liver cancer (Table 2).
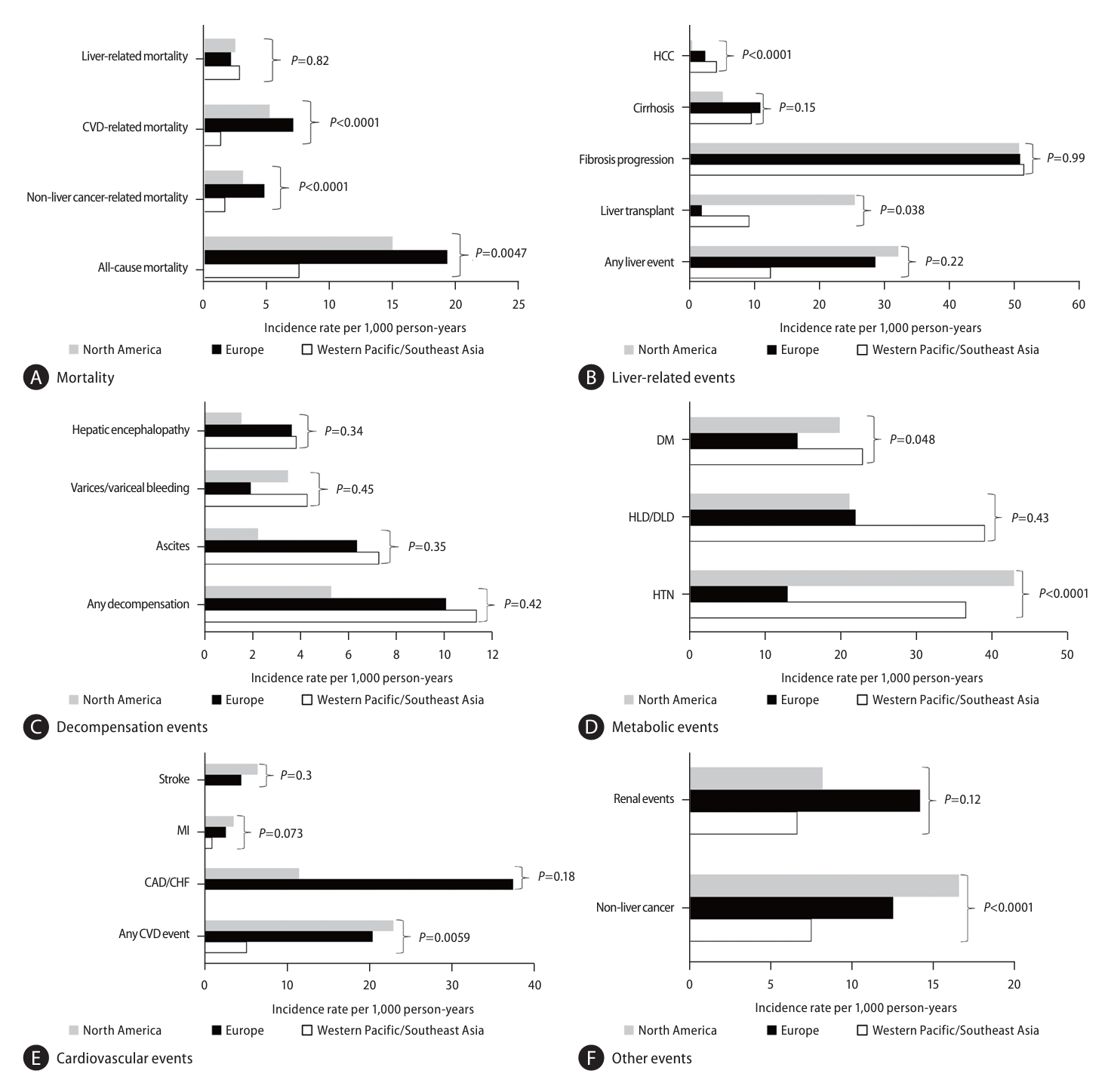
Significantly higher rates of all-cause, CVD-related, and non-liver cancer-related mortality were observed in Europe and North America compared to Western Pacific/Southeast Asia with no differences observed for liver-related mortality (Fig. 2A). Significantly higher incidence of HCC was observed in Western Pacific/Southeast Asia compared to North America and Europe (Fig. 2B), though no differences were observed for other liver-related events (Fig. 2C).
For non-liver events, pooled incidence of CVD and HTN as well non-liver events were highest in North America while Western Pacific/Southeast Asia had the lowest rates for both CVD and non-liver cancer (P=0.0059, <0.001, and <0.001, respectively, Fig. 2CŌĆōF, Supplementary Table 4).
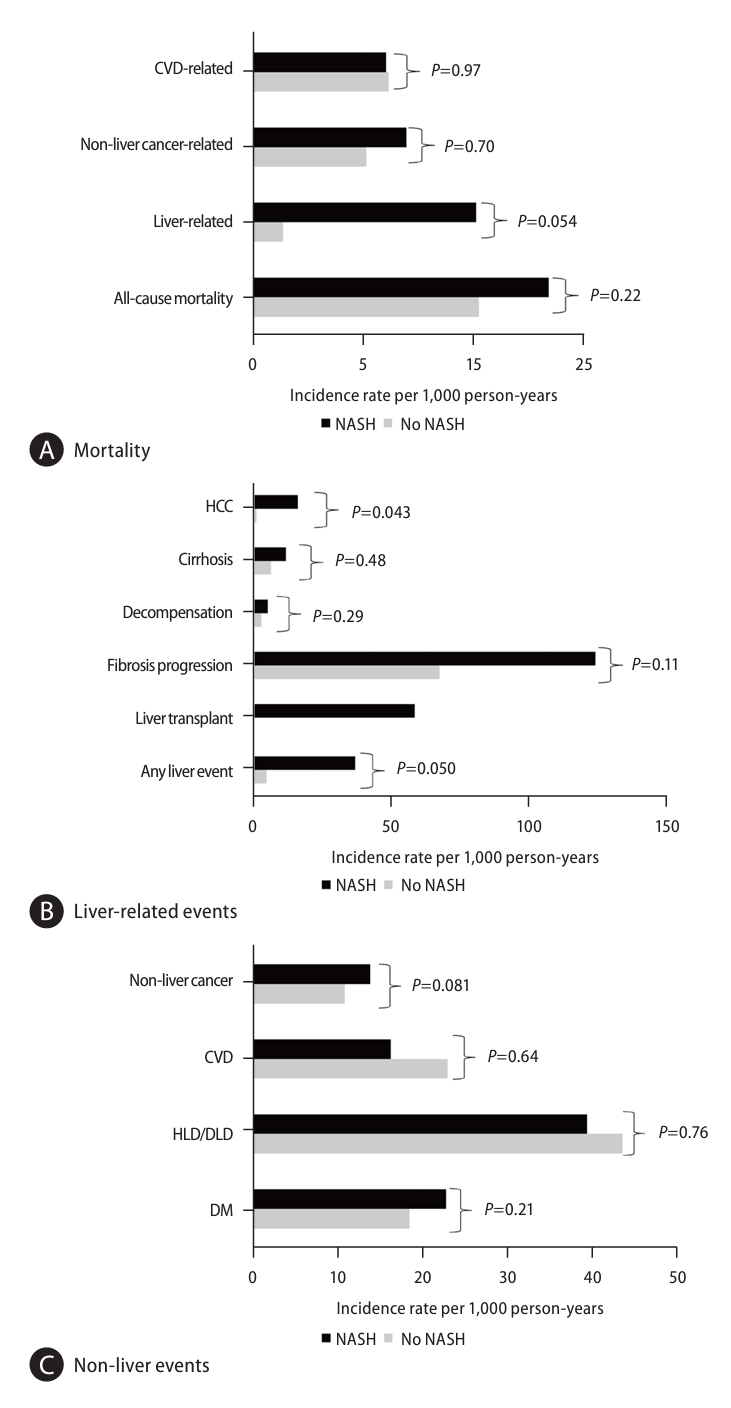
Analysis of 14,570 non-NASH NAFLD diagnosed via biopsy and 9,297 patients with biopsy-proven NASH showed those with NASH had a significantly higher incidence of HCC compared to those without NASH (P=0.04, Supplementary Table 5 and Fig. 3AŌĆōC). No statistically significant differences for liver-related and non-liver mortality (all P>0.05).
Those with biopsy-diagnosed NAFLD had the highest rates of mortality (P=0.0039), fibrosis progression (P=0.0004), and liver transplant (P=0.0096) compared to NAFLD diagnosed via ultrasound, ICD, and other imaging. HCC incidence was also significantly higher among those with biopsy-diagnosed NAFLD compared to NAFLD diagnosed by ultrasound or ICD codes (P=0.0002). Meanwhile, NAFLD diagnosed via ultrasound had higher incidence of CAD/CHF and stroke compared to biopsy-diagnosed NAFLD (Supplementary Table 6).
There were no statistically significant differences in the pooled incidence of adverse clinical events among studies with a baseline prevalence of fibrosis less than the median (57%) and studies with a baseline prevalence of advanced fibrosis greater than the median (Supplementary Table 8). On the other hand, studies with a baseline prevalence of cirrhosis greater than the median (17%) had higher incidence of liver-related mortality, liver-related events, and HCC (Supplementary Table 9).
By the median study year of 2007, there were significant decreases in the incidence rates of cardiovascular-related mortality (P<0.0001), non-liver cancer related mortality (P<0.0001), and non-liver cancer (P=0.0012), with a trending decrease in all-cause mortality (P=0.0648). However, there were significant increases in incidence rates of decompensated cirrhosis (P=0.022) (Supplementary Table 7). Notably, there was a decrease in baseline median age of the cohort by the study year, with a regression coefficient of 0.19 per year (95% confidence interval 0.01ŌĆō0.37) P=0.039.
No significant differences in outcomes were noted between males and females with NAFLD (Supplementary Fig. 3).
In this study, using pooled data from 79 studies involving almost 1.4 million persons, we estimated the incidence rates for a comprehensive range of adverse events to include mortality, liver, and various non-liver outcomes for patients with NAFLD. The estimated pooled incidence rate for all-cause mortality was 14.6 per 1,000 person-years. Among the cause-specific mortality, the rates were high for both liver as well as non-liver mortality (4.5, 4.5, and 3.1 per 1,000 person-years for cardiovascular, non-liver cancer, and liver related causes). This finding helps confirm previous reports that cardiovascular disease is among the primary causes of death among those with NAFLD especially among those with significant liver fibrosis or cirrhosis [23,24].
In addition to mortality, we also estimated incidence rates per 1,000 person-years for liver-related events which were 24.3 overall, with notably lower rate of HCC (3.4) compared to other liver events (49.0 for fibrosis progression, 10.9 for cirrhosis, and 12.0 for liver transplant). The overall event rate for cardiovascular disease (24.8) was like that of liver-related events, and the non-liver cancer rate (10.5) was also similar to that of cirrhosis, while the rates of renal and depression/anxiety incidence were both about 30%. We estimated high incidence of cardiovascular events among those with NAFLD, especially CAD/CHF at a rate of 40 per 1,000 person-years. These findings further confirm the high cardiovascular burden of NAFLD identified by a prior meta-analysis on cardiovascular events [25]. This current study further reinforces the significant associations between NAFLD and kidney disease that have been identified in the literature [26,27]. A prior meta-analysis of middle-aged persons found a 1.2-1.5-fold increased risk of extrahepatic cancer among those with NAFLD [28]. This current study further confirms these findings with an estimated incidence rate of 10.5 per 1,000 person-years among those with NAFLD. As this current study identified only one study on the incidence of depression/anxiety among those with NAFLD, further studies should be considered to better understand this association. We estimated the incidence of metabolic events to be high at a rate of approximately 20 per 1,000 person-years for DM, HLD/DLD, HTN, and MetS. Given that MetS is a major factor in the progression from NAFLD to NASH and significantly associated with increased risk for mortality, preventing development of MetS via a multidisciplinary approach is vital towards preventing both liver and non-liver adverse events among those with NAFLD [29,30].
In our sub-analyses, we identified several differences in outcomes by region between North America, Europe, and Western Pacific/Southeast Asia. Compared to Europe and North America, Western Pacific/Southeast Asia had a lower risk for all-cause, cardiovascular-related, and non-liver cancer related mortality; however, there were no significant differences between the regions for liver-related mortality. Though non-liver cancer development was lower in Western Pacific/Southeast Asia, incidence of HCC was significantly higher compared to Europe and North America. Such differences in cardiovascular and non-liver cancer related mortality may be explained by the prevalence of the western diet in Europe and North America while the higher incidence of HCC development may be related to the higher prevalence of the PNPLA3 gene polymorphism in East Asia and its role in the development of HCC [31,32]. Furthermore, the higher risk of HCC in Western Pacific/Southeast Asia may be due to competing risks for this outcome, as this population was found to also have the lowest risks for CVD-related, non-liver cancer-related, and all-cause mortality, thus allowing this population to live long enough to develop HCC. We excluded studies that included patients with known chronic hepatitis B but since hepatitis B virus is endemic in Asia, patients with NAFLD may have undiagnosed or occult HBV that can add to the risk for HCC development.
From the liver disease standpoint, non-NASH is felt to be mostly non-progressive whereas NASH can progress to cirrhosis and is not benign. We also explored incident events stratified by biopsy-diagnosed NASH compared to non-NASH patients. Interestingly, only liver-related mortality approached being significantly higher in those with NASH (10.2 per 1,000 person-years) compared to non-NASH patients (1.4 per 1,000 person-years P=0.0503). On the other hand, those with NASH had a significantly higher incidence rate of HCC (14.8 per 1,000 person-years) compared to the non-NASH patients (0.79 per 1,000 person-years, P=0.04). The inability to find a significant difference in mortality and other events may be due to the non-significant difference in the incidence of fibrosis progression between the two groups. The leading predictor of adverse outcomes appears to be the progression of fibrosis. However, this suggestion needs further study as there was only one study used that reported on fibrosis progression [33]. Additionally, as these studies included biopsy-diagnosed NAFLD, the study population likely had selection bias and may include those who are sicker and thus predisposed to adverse outcomes. As such, the results may underestimate the true difference in outcomes between NASH and non-NASH NAFLD. This is further evidenced in the comparison of adverse events by diagnostic modality, which found overall significantly higher rates of events, specifically liver-related events, among those diagnosed via biopsy compared to ultrasound; there could be misclassification in the diagnosis without liver biopsy. On the other hand, those that had NAFLD diagnosed via ultrasound had higher incidence rates for CAD/CHF and stroke, suggesting that NAFLD may have been found incidentally [34]. Another important finding is the decreasing rates of non-liver events such as cardiovascular and non-liver cancer related events while liver-related events have increased between the pre-2007 and the post-2007 time-period, which may be due to a longer history of preventive efforts for cardiovascular disease and the cohort effect of the emerging NAFLD epidemic. The decreasing age of the study cohorts in the pre-2007 as compared to the post-2007 time period also suggests the effect of the increased awareness and diagnosis of NAFLD during the past three decades as well as the increasing prevalence of metabolic syndrome globally. Stratified analysis by sex found no significant differences in adverse events. Additionally, there was increased incidence of liver-related events, HCC, and liver-related mortality among studies with higher baseline prevalence of cirrhosis though similar association was not statistically significant among studies with higher prevalence of advanced fibrosis. Together these results validate and extend the results from a recently published meta-analysis [35].
A strength of our study is that we identified studies from multiple different regions. However, given that we only identified studies from Europe, North America, and Western Pacific/Southeast Asia, this current study does not include studies from Africa or South America, where NAFLD prevalence may be much higher [2]. As such, further studies should be considered from other regions to further our understanding of the adverse effects of NAFLD on these populations. A limitation is that we did not include NAFLD diagnosed via non-invasive blood-based methods. However, given the non-standardized cutoffs across different populations and the fact that imaging is the recommended modality for NAFLD diagnosis, we felt it reasonable to include only those studies diagnosed via imaging or ICD-code [36]. Additionally, as several studies did not report the person-years of follow-up, we used mean/median years follow-up to estimate the total person-years follow-up. This may have overestimated the denominator for the incidence rate, therefore underestimating the true incidence rate of adverse events associated with NAFLD. Despite non-significant EggersŌĆÖ test, DerSimonian-Laird and Sidik-Jonkman random-effects model adjustments, publication bias may remain and should be taken into consideration when interpreting this data. The results of our subgroup analysis identified that the effects of heterogeneity on incidence of adverse events rely on multiple factors across studies including region and baseline health status. Subgroup data should be interpreted with caution as data were not available for all studies and sample size was smaller, thus leading to the potential introduction of additional bias. Additionally, we caution the interpretation of the results given the difficulty of confirming the temporal relationship between NAFLD and metabolic events as some patients may have had undiagnosed metabolic dysfunction at baseline, especially in those patients from retrospective cohort studies. Genetic polymorphism and the degree of severity as well as medical control of metabolic comorbidities may also have played a significant role in the development of MASLD, and should be examined in future studies. Finally, despite the minimal discrepancies between NAFLD and MASLD, the cautious interpretation of the results will be needed as the evaluation of outcomes associated with MASLD awaits future studies in this newly proposed disease category [18-20].
To our knowledge, this is the first meta-analysis that identified the incidence rates of adverse events associated with NAFLD for a wide range of outcomes with detailed subgroup data. We estimated the incidence rates for mortality, liver-related events, non-liver related events, and provided estimates stratified by sex, baseline NASH status, diagnostic modality, time-period, and world regions. These incidence rates can be used to implement proper interventions targeted towards prevention and treatment, which is imperative as the prevalence of NAFLD increases. With the proposed changes in diagnostic criteria and nomenclature of NAFLD to MASLD, this study should guide future research on outcomes associated with MASLD.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary┬ĀTable┬Ā2.
Quality assessment of included studies
Supplementary┬ĀTable┬Ā4.
Clinical outcomes among NAFLD participants by region
Supplementary┬ĀTable┬Ā5.
Clinical outcomes among NAFLD participants with liver biopsy, stratified by biopsy-confirmed NASH
Supplementary┬ĀTable┬Ā6.
Clinical outcomes among NAFLD participants, stratified by NAFLD diagnostic method
Supplementary┬ĀTable┬Ā7.
Clinical outcomes among NAFLD participants, by median study year
Supplementary┬ĀTable┬Ā8.
Incidence of adverse clinical events, stratified by study prevalence of advanced fibrosis
Supplementary┬ĀTable┬Ā9.
Incidence of adverse clinical events, stratified by study prevalence of cirrhosis
Supplementary┬ĀFig.┬Ā2.
Forest plot for analyses of clinical outcomes of NAFLD participants
Supplementary┬ĀFig.┬Ā3.
Clinical outcomes in NAFLD participants stratified by sex
FOOTNOTES
Figure┬Ā1.
Study selection flowchart and subgroups by NAFLD diagnostic method. NAFLD, nonalcoholic fatty liver disease.
Figure┬Ā2.
Clinical outcomes among NAFLD participants stratified by region (see corresponding forest plots in Supplementary Figure 2AŌĆōM and additional details in Supplementary Table 4). (A) Mortality, (B) Liver-related events, (C) Decompensation events, (D) Metabolic events, (E) Cardiovascular events, (F) Other events. NAFLD, nonalcoholic fatty liver disease; CVD, cardiovascular disease; DM, diabetes mellitus; HLD/DLD, hyperlipidemia/dyslipidemia; HTN, hypertension; MI, myocardial infarction; CAD/CHF, coronary artery disease/congestive heart failure.
Figure┬Ā3.
Clinical outcomes among NAFLD participants with liver biopsy, stratified by biopsy proven NASH (additional details in Supplementary Table 5). (A) Mortality, (B) Liver-related events, (C) Nonliver events. NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CVD, cardiovascular disease; HCC, hepatocellular carcinoma; HLD/DLD, hyperlipidemia/dyslipidemia; DM, diabetes mellitus.
Table┬Ā1.
Baseline characteristics among participants with NAFLD
Table┬Ā2.
Incidence rate of adverse events among participants with baseline NAFLD
| Events | Studies (n) | Participants (n) | Events (n) | Incidence rate per 1,000 person-years (95% CI) | I2 (%)* |
|---|---|---|---|---|---|
| Mortality | |||||
| ŌĆāAll-cause | 37 | 513,409 | 75,371 | 14.57 (11.45ŌĆō17.69) | 99.9 |
| ŌĆāCardiovascular disease-related | 23 | 116,656 | 2,240 | 4.53 (2.92ŌĆō6.15) | 99.0 |
| ŌĆāNon-liver cancer related | 22 | 116,949 | 2,239 | 3.27 (2.38ŌĆō4.15) | 98.7 |
| ŌĆāLiver-related | 26 | 118,005 | 912 | 3.10 (1.96ŌĆō4.24) | 97.0 |
| Liver-related events | |||||
| ŌĆāAny event | 40 | 603,907 | 108,512 | 24.28 (13.98ŌĆō34.58) | 100.0 |
| ŌĆāFibrosis progression | 5 | 1,098 | 253 | 48.95 (22.86ŌĆō75.04) | 92.8 |
| ŌĆāCirrhosis (compensated + decompensated) | 21 | 309,694 | 5,370 | 10.85 (6.65ŌĆō15.06) | 99.5 |
| ŌĆāLiver transplant | 10 | 3,503 | 126 | 11.99 (0.00ŌĆō28.26) | 92.3 |
| ŌĆāHCC | 30 | 336,845 | 1,025 | 3.39 (1.97ŌĆō4.81) | 94.6 |
| Cirrhotic decompensation | |||||
| ŌĆāAny decompensation | 16 | 131,983 | 3,136 | 12.08 (6.80ŌĆō17.36) | 98.3 |
| ŌĆāAscites | 13 | 4,764 | 201 | 6.68 (2.75ŌĆō10.61) | 90.7 |
| ŌĆāVarices/variceal bleeding | 11 | 4,318 | 139 | 4.72 (2.57ŌĆō6.87) | 83.0 |
| ŌĆāHepatic Encephalopathy | 8 | 3,462 | 58 | 2.31 (1.07ŌĆō3.55) | 72.0 |
| Metabolic outcomes (in people without metabolic disease at baseline) | |||||
| ŌĆāMetabolic syndrome | 3 | 535 | 151 | 25.40 (0.00ŌĆō68.87) | 98.6 |
| ŌĆāHypertension | 12 | 5,591 | 922 | 25.84 (14.47ŌĆō37.21) | 98.4 |
| ŌĆāHyperlipidemia/Dyslipidemia | 7 | 9,549 | 885 | 26.43 (11.38ŌĆō41.47) | 98.4 |
| ŌĆāDiabetes mellitus* | 24 | 39,562 | 5,025 | 19.01 (15.01ŌĆō23.03) | 98.4 |
| Cardiovascular events (in people without cardiovascular disease at baseline) | |||||
| ŌĆāAny event | 19 | 188,368 | 11,222 | 24.77 (8.46ŌĆō41.08) | 99.8 |
| ŌĆāCoronary artery disease/congestive heart failure | 8 | 27,706 | 3,695 | 40.12 (2.13ŌĆō78.11) | 98.3 |
| ŌĆāMyocardial infarction | 9 | 185,174 | 1,974 | 7.07 (0.00ŌĆō14.90) | 99.0 |
| ŌĆāStroke (ischemic/hemorrhagic) | 10 | 148,571 | 3,602 | 8.05 (0.51ŌĆō15.59) | 92.5 |
| Renal events | 6 | 330,872 | 14,712 | 30.28 (0.00ŌĆō70.87) | 99.8 |
| ŌĆāDepression/anxiety | 1 | 19,871 | 5,783 | 29.10 (28.35ŌĆō29.85) | N/A |
| ŌĆāNon-liver cancer | 11 | 29,243 | 3,165 | 10.49 (8.41ŌĆō12.56) | 96.5 |
Abbreviations
CLD
chronic liver disease
NAFLD
nonalcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
DM
type 2 diabetes mellitus
HTN
hypertension
HLD/DLD
hyperlipidemia/dyslipidemia
HCC
hepatocellular carcinoma
MetS
metabolic syndrome
CVD
cardiovascular disease
BMI
body mass index
CAD/CHF
coronary artery disease/congestive heart failure
MASLD
metabolic dysfunction-associated steatotic liver disease
REFERENCES
2. Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022;20:2809-2817.e28.


3. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol 2021;6:578-588.


4. Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology 2018;67:1726-1736.




5. Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 2017;12:e0173499.



6. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608-612.


7. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD. Hepatology 2020;72:1605-1616.



8. Gonzalez-Chagolla A, Olivas-Martinez A, Ruiz-Manriquez J, Serv├Łn-Rojas M, Kauffman-Ortega E, Ch├Īvez-Garc├Ła LC, et al. Cirrhosis etiology trends in developing countries: Transition from infectious to metabolic conditions. Report from a multicentric cohort in central Mexico. Lancet Reg Health Am 2021;7:100151.



9. Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580-589.e5.


10. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748-755.e3.


11. Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014;60:110-117.


12. Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther 2017;46:856-863.



13. Younossi ZM, Tampi RP, Racila A, Qiu Y, Burns L, Younossi I, et al. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care 2020;43:283-289.



14. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577-1586.



15. Le MH, Yeo YH, Zou B, Barnet S, Henry L, Cheung R, et al. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin Mol Hepatol 2022;28:841-850.




16. World Obesity Federation. World Obesity Atlas 2023. World Obesity Federation web site, <https://data.worldobesity.org/publications/?cat=19>. Accessed 10 Jun 2023.
17. Younossi ZM. Patient-reported outcomes and the economic effects of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: the value proposition. Hepatology 2018;68:2405-2412.



18. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542-1556.

19. Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol 2024;80:e54-e56.

20. Lee BP, Dodge JL, Terrault NA. National prevalence estimates for steatotic liver disease and subclassifications using consensus nomenclature. Hepatology 2024;79:666-673.


21. Xiao J, Ng CH, Chan KE, Fu C, Tay P, Yong JN, et al. Hepatic, extra-hepatic outcomes and causes of mortality in NAFLD - An umbrella overview of systematic review of meta-analysis. J Clin Exp Hepatol 2023;13:656-665.


22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.



23. Ekstedt M, Hagstr├Čm H, Nasr P, Fredrikson M, St├źl P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-1554.


24. Taylor RS, Taylor RJ, Bayliss S, Hagstr├Čm H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 2020;158:1611-1625.e12.


25. Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021;6:903-913.


27. Roderburg C, Krieg S, Krieg A, Demir M, Luedde T, Kostev K, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of chronic kidney disease (CKD). Eur J Med Res 2023;28:153.




28. Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022;71:778-788.


29. Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore) 2018;97:e0214.



30. Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol 2021;19:2172-2181.e6.


31. Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol 2021;74:775-782.



32. Yip TC, Lee HW, Chan WK, Wong GL, Wong VW. Asian perspective on NAFLD-associated HCC. J Hepatol 2022;76:726-734.


33. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559-1569.



34. Mishra S, Bhujade H, Butt AS, Kamani L, Premkumar M. Work-up for Incidentally Detected NAFLD: How Far is It Worth? Euroasian J Hepatogastroenterol 2022;12(Suppl 1):S26-S36.



APPENDICES
Appendix.
NEWCASTLE - OT TAWA QUALIT Y ASSESSMENT SCALE COHORT STUDIES
Selection
1) Representativeness of the NAFLD cohort
ŌĆāa) truly representative of the average population in the community (2*)
ŌĆāb) somewhat representative of the average population in the community (1*)
ŌĆāc) selected group of users eg nurses, volunteers
ŌĆād) no description of the derivation of the cohort
3) Ascertainment of baseline NAFLD status
ŌĆāa) secure record (1*)
ŌĆāb) structured interview (1*)
ŌĆāc) self-report
ŌĆād) no description
Comparability
1) Comparability of cohorts on the basis of the design or analysis
ŌĆāa) study controls for sex (2*)
ŌĆāb) study controls for other additional factors: obesity, NASH (2*)
ŌĆāc) study does not control for any factors
Outcome
1) Assessment of outcome among those with NAFLD
ŌĆāa) record linkage (1*)
ŌĆāc) noninvasive testing
ŌĆād) self-report
ŌĆāe) no description
2) Was follow-up long enough for outcomes to occur
ŌĆāa) yes, >5 years (1*)
ŌĆāb) no, Ōēż5 years
3) Adequacy of follow up of cohorts
ŌĆāa) complete follow up - all subjects accounted for (1*)
ŌĆāb) subjects lost to follow up unlikely to introduce bias - small number lost - <20% (1*)
ŌĆāc) follow up rate < 80% and no description of those lost
ŌĆād) no statement
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print





