| Clin Mol Hepatol > Volume 18(4); 2012 > Article |
ABSTRACT
Background/Aims
This study evaluated the clinical outcomes of balloon-occluded retrograde transvenous obliteration (BRTO) for the treatment of hemorrhage from gastric varices (GV) in Korean patients with liver cirrhosis (LC).
Methods
We retrospectively analyzed data from 183 LC patients who underwent BRTO for GV bleeding in 6 university-based hospitals between January 2001 and December 2010.
Results
Of the 183 enrolled patients, 49 patients had Child-Pugh (CP) class A LC, 105 had CP class B, and 30 had CP class C at the time of BRTO. BRTO was successfully performed in 177 patients (96.7%). Procedure-related complications (e.g., pulmonary thromboembolism and renal infarction) occurred in eight patients (4.4%). Among 151 patients who underwent follow-up examinations of GV, 79 patients (52.3%) achieved eradication of GV, and 110 patients (72.8%) exhibited marked shrinkage of the treated GV to grade 0 or I. Meanwhile, new-appearance or aggravation of esophageal varices (EV) occurred in 54 out of 136 patients who underwent follow-up endoscopy (41.2%). During the 36.0┬▒29.2 months (mean┬▒SD) of follow-up, 39 patients rebled (hemorrhage from GV in 7, EV in 18, nonvariceal origin in 4, and unknown in 10 patients). The estimated 3-year rebleeding-free rate was 74.8%, and multivariate analysis showed that CP class C was associated with rebleeding (odds ratio, 2.404; 95% confidence-interval, 1.013-5.704; P=0.047).
Although the risk of bleeding from gastric varices (GV) is relatively low, once GV rupture occurs, the outcome is worse, with a higher mortality than esophageal varices (EV).1,2 For the treatment of GV bleeding, various treatment modalities including endoscopic procedures, interventional radiologic treatment, and surgery have been widely performed.3-5 Balloon-occluded retrograde transvenous obliteration (BRTO) is an interventional radiologic treatment option that was introduced in 1990s, by Kanagawa et al.6 This procedure involves occlusion of blood flow by inflation of a balloon catheter into an outflow shunt, such as a gastro-renal shunt, and injection of ethanolamine oleate into GV in a retrograde manner.
Because BRTO is less invasive and relatively safe than other treatment options, it has been becoming the effective treatmentchoice for GV bleeding in patients whose hemodynamics allow its application, and has been widely performed in Japan,6-13 and Korea.14-17 However, there is little known of the long-term outcomes of BRTO in Korea. The purpose of this study is, therefore, to retrospectively investigate the clinical outcomes of BRTO for the treatment of GV bleeding in Korean patients with liver cirrhosis (LC).
This retrospective review of patients' medical and imaging records was approved by each institutional review board. A total of 183 patients with liver cirrhosis (LC) who underwent BRTO for the treatment of endoscopically confirmed GV bleeding at Kyungpook National University Hospital, Konkuk University Hospital, Soonchunhyang University Bucheon Hospital, Samsung Medical Center, Kangbook Samsung Hospital, and Hanyang University Guri Hospital, in Korea, between January 2001 and December 2010, were enrolled in this study. No enrolled patients received other endoscopic, surgical or radiologic interventional treatments prior to BRTO. In each patient, GV were confirmed by esophagogastroduodenoscopy (EGD), and gastrorenal shunt was demonstrated by contrast-enhanced computer tomography (CT). GV were classified by anatomic distribution as proposed by Sarin et al.1,18 The sizes of GV were classified according to the system suggested by Hashizume et al19 as follows: grade 0, non visible; grade I, small sized, tortuous winding varices; grade II, medium sized, nodular-shaped varices; grade III, large sized, tumorous GV. The sizes of esophageal varices (EV) were graded according to the following classification20: grade 0, not visible; grade I, varices occupying less than 25% of the lumen; grade II, varices occupying 25 to 50% of the lumen; grade III, varices occupying greater than 50% of the lumen. Technical success of BRTO was arbitrarily defined as the completion of the procedure after the confirmation of sufficient obliteration of GV with sclerosant under retrograde venography, and without re-bleeding at 2 days after BRTO. Procedure-related complications were defined as any untoward adverse events during or immediately after BRTO, which required active treatment or prolonged hospitalization. Eradication of GV was considered as complete obliteration of targeted GV on follow-up CT or EGD. Rebleeding was regarded as the presence of hematemesis, melena, or hematochezia with a significant drop in hemoglobin level and blood transfusion of two or more units was needed. If the bleeding source was proven endoscopically to originate from GV or EV, it was considered variceal re-bleeding. Patients who were lost to follow up were right-censored at the time of drop out. Patients who failed to BRTO were excluded for the analysis of the rebleeding and the mortality rates.
In each center, BRTO was performed according to the method similar to that described by Kanagawa.6 In brief, a catheter for BRTO was inserted through the femoral or internal jugular vein, and then, it was advanced toward the gastrorenal shunt through the left renal vein and subsequently placed in the proximal portion of a shunt by ballooning. Venography was performed to demonstrate GV, gastrorenal shunts, and collateral veins communicating with the shunt. Ethanolamine oleate mixed with lipiodol was then injected to obliterate GV until there was sufficient accumulation of the mixture in GV. Finally, after confirmation that blood flow through the catheter had ceased, the balloon catheter was carefully deflated and removed under fluoroscopic observation while closely observing whether the formed thrombus remained stationary.
The data are expressed as the mean┬▒standard deviation (SD) or median with range. Quantitative variables were compared using Student's t-test, and qualitative variables were compared using Fisher's exact test. Kaplan-Meier analyses were applied to examine the time to the first episode of re-bleeding, or time to death. The log-rank test was used to examine the variation of rebleeding episodes and survival rates. Age, sex and variables with P<0.15 in univariate analysis were subjected to multivariate Cox regression modelling using forward stepwise variable selection. All P-values were two- tailed, and a P-value less than 0.05 was considered significant. Analyses were performed using SPSS software version 11.5 (SPSS, Chicago, IL, USA).
The patients included 139 men and 44 women with a mean age of 57.1┬▒9.9 years. All patients had underlying liver cirrhosis, of which the causes were hepatitis B (n=90), hepatitis C (n=23), chronic alcohol abuse (n=50), others (including autoimmune hepatitis, primary biliary cirrhosis, cryptogenic, etc) (n=13), and combined etiologies (n=7). According to the Child-Pugh classification (CP class), 48 patients were class A, 103 patients were class B, and 32 patients were class C. The mean pre-procedural Child-Pugh score was 7.72┬▒1.87. Fifty patients had coexisting viable hepatocellular carcinoma (HCC) at the time of BRTO. According to the Barcelona Clinic Liver Cancer (BCLC) stage, 2 patients were classified as Stage 0, 19 as stage A, 15 as stage B, 12 as stage C, and 2 as stage D. The endoscopic locations of GV were isolated gastric varices type 1 (IGV1) in 61 patients, gastroesophageal varices type 1 (GOV1) in 42, and gastroesophageal varices type 2 (GOV2) in 80 patients. The sizes of GV were grade I in 18 patients, grade II in 40 patients, grade III in 124 patients, and undetermined in 1 patient. 131 patients had concomitant EV at the time of BRTO, and their sizes were grade I in 87 patients, grade II in 38 patients, and grade III in 6 patients. The characteristics of patients were summarized in Table 1.
Technical success of BRTO was achieved in 177 of 183 patients (96.7%). Procedure-related complications (during or immediately after BRTO) occurred in 8 patients (4.4%). Pulmonary thromboembolism developed in 5 patients, and three cases of them were associated with balloon rupture during BRTO procedure. Transient mental change, left renal infarction, and gastrorenal shunt rupture each occurred in one patient.
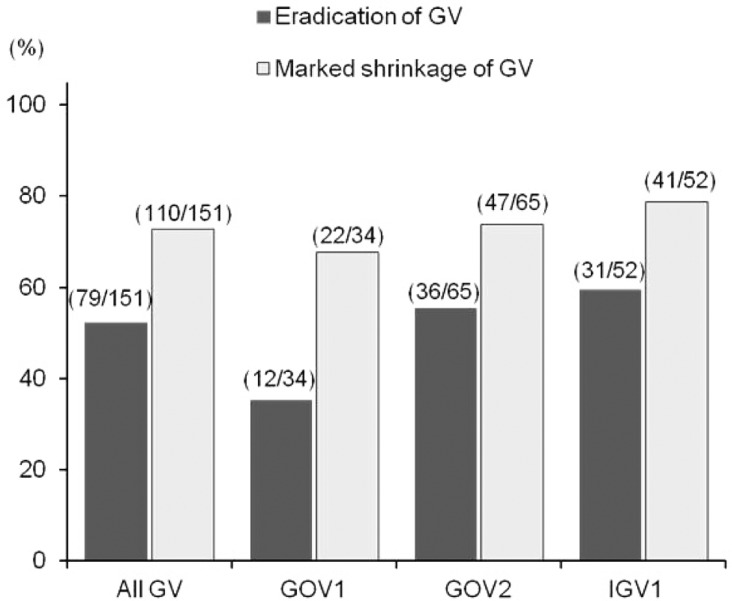
Of 177 patients who successfully completed BRTO, 151 patients had the endoscopic or radiologic follow-up examinations (136 patients with EGD, 15 patients with only CT). Among these, 79 patients (52.3%) achieved eradication of GV, 110 patients (72.8%) showed marked shrinkage of treated GV to grade 0/I, and 129 patients (85.4%) showed decrease in size of GV after BRTO. When analyzing the eradication of GV after BRTO in terms of location of GV, the eradication rates of GOV1, GOV2 and IGV1 were 35.5% (12/34), 55.4% (36/65), and 59.6% (31/52) respectively (Fig. 1), and, the eradication rate of GOV1 was significantly lower than IGV1/GOV2 (P=0.032).
EV was newly developed in 21 of 36 patients without EV prior to BRTO (58.3%), and EV progressed to a larger size in 33 of 100 patients with EV prior to BRTO (33.0%) (Fig. 2). On the follow-up EGD findings, 57 patients had grade II/III EV after BRTO (41.9%), and 35 patients of them underwent prophylactic endoscopic variceal ligation (EVL).
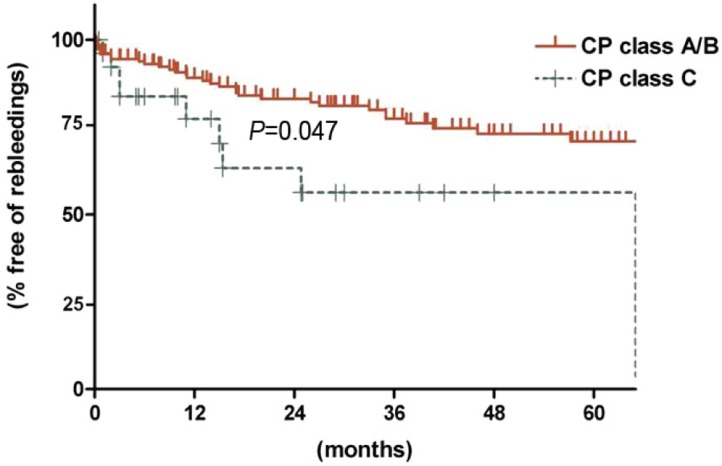
During the follow-up period after BRTO (mean, 36.0┬▒29.2 months), re-bleedings from gastrointestinal tracts occurred in 39 patients (22.0%). Among these, bleedings from EV and GV occurred in 18 and 7 patients, respectively. Non-variceal bleedings occurred in 4 patients (benign gastric ulcer, duodenal ulcer, portal hypertensive gastropathy). In 10 patients, bleeding sources couldn't be evaluated (Fig. 3). The estimated 1-, 3- and 5- year rebleeding-free rates were 87.5%, 74.8%, and 68.9% respectively. Of potential variables, such as gender, age (>55 vs. Ōēż55 years), presence of HCC, BCLC stage (B/C/D vs. no HCC/0/A), CP class (class A/B vs. class C), etiology of LC (alcohol vs. non-alcohol), location of GV (GOV1 vs. GOV2/IGV1), size of GV (grade I vs. grade II/II or grade I/II vs. grade III), size of EV (grade 0/I vs. grade II/II), or eradication of GV, CP class C and presence of advanced stage HCC (BCLC B/C/D) were significantly or marginally associated with re-bleeding on univariate analysis. On multivariate analysis, only CP class C was significantly associated with re-bleeding (odds ratio [OR], 2.404; 95% confidence interval [CI], 1.013-5.704; P=0.047) (Table 2, Fig. 4).
At the end of follow-up, 52 patients were dead, and their causes of death were HCC (n=15), re-bleeding (n=13), hepatic failure (n=6), complications of portal hypertension except variceal bleedings (hepatic encephalopathy, spontaneous bacterial peritonitis [SBP], hepatorenal syndrome, etc) (n=7), infections except SBP (pneumonia, etc) (n=3) and others (n=8). The estimated 1-, 3-, 5- year overall survival rates were 86.2%, 71.0%, and 65.8% respectively. Of potential variables, presence of HCC (P=0.001) and Child-Pugh class C (P=0.022) were significantly associated with poor survival (Fig. 5). On multivariate analysis, presence of HCC finally remained independently correlated with poor prognosis (OR, 2.897; 95%CI 1.612-5.204, P=0.001) (Table 2).
Despite the minimal invasiveness and high effectiveness of BRTO, it was not mentioned for the treatment of GV in the U.S. (American Association for the Study of Liver Diseases, AASLD) or European (Baveno V) guidelines.21,22 Recently, clinical practice guidelines for liver cirrhosis by the Korean Association for the Study of the Liver (KASL),23 suggested that BRTO could be considered for the treatment of GV with gastrorenal shunt. However, they also mentioned that its strength of evidence was not strong. Therefore, we proposed to show the safety, efficacy and long-term clinical outcomes of BRTO for the treatment of GV bleeding, even though our study was retrospectively designed.
In this study, BRTO could be successfully performed without failure in almost patients, and the procedure was relatively safe for the treatment of GV. Our study showed that the technical success rate was 96.7% with 4.4% procedure-related complications, similar compared to the previous results, mainly reported in Japan.8,10,12 Despite the positive results of our study, however, it is difficult to recommend BRTO as the first choice treatment in the setting of an active GV bleeding, because the presence of gastrorenal shunt should be identified before the procedure of BRTO, and BRTO is a time-consuming procedure compared to endoscopic management. Therefore BRTO seems to be the treatment of choice for patients whose GV bleedings are not active (spurting or oozing), or secondary prophylaxis for prevention of GV re-bleeding.
From the perspective of GV eradication, our results showed relatively lower eradication rate of treated GV (52.3%), compared to the previous reported data ranging from 80-100%.8-10,12,17 However, considering that about three fourths of patients showed marked shrinkage of the treated GV, and among these, a only few experienced re-bleeding, BRTO might be the clinically effective treatment of GV. Interestingly, eradication rate of GOV1 with BRTO was significantly lower than GOV2/IGV1. Theoretically, GV represent a heterogeneous entity anatomically and hemodynamically. They are commonly categorized on the basis of their location in the stomach and their relationship with EV.1,18 Among these, GOV1 are the most common subtype of GV and constitute an extension of EV along the lesser curvature of the stomach. Because they are considered a continuation of EV, KASL suggested that GOV1 should be treated as EV, and BRTO was not suitable for the management of GOV1. We guess our data supported their suggestions, and GOV1 might be treated with other treatment modality rather than BRTO, even though gastrorenal shunt exists in those cases.
As it is known in common, the major drawback of BRTO is potential worsening of EV.8,9,14,16 The worsening rate has previously reported in up to 66% of patients, probably because of the increased portal flow and pressures. Our data also showed that new EV appeared in more than one-half of patients who had no EV prior to BRTO, and EV was aggravated in about one-third of patients with EV prior to BRTO. Moreover, the incidence of EV bleeding (18 cases) is higher than that of GV re-bleeding (7 cases). Therefore, close monitoring with/without prophylaxis of EV should be necessary after BRTO.
In this study, pre-procedural liver functional reserve was the major factor which affected the re-bleeding and prognosis of patients undergoing BRTO. CP class C was a significant independent factor for re-bleeding on multivariate analysis, and was marginally associated with dismal prognosis of patients. However, several reports demonstrated that BRTO could improve liver function in LC patients with portal hypertension, probably because of increased portal flow.11,24,25 Therefore, the benefits and risks from this procedure might be weighed for patients whose liver functional reserve is already profoundly compromised.
There are several limitations in this study. First, because this is a retrospective study, BRTO might be performed in patients selected according to the protocol of each medical center. Second, because the reviewers of endoscopic or radiologic images were different in each center, inter-observer variations might exist, even though all centers used the same classification or grading system as mentioned in methods section. Third, we couldn't evaluate whether BRTO could improve liver functional reserve or hepatic encephalopathy. Several reports suggested that the BRTO could improve not only GV, but also hepatic functional reserve or hepatic encephalopathy, and these might be other merits of BRTO.11,24-26 In spite of the limitations, there were a sizable number of patients enrolled in this study, and they were followed-up for a long time period.
In conclusions, BRTO can be performed safely and effectively for the treatment of GV bleeding. But occurrence/progression of EV or bleeding from EV is not uncommon after BRTO. Periodic endoscopy to follow-up EV with/without prophylactic treatment might be needed in LC patients undergoing BRTO.
REFERENCES
1. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a longterm follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343-1349. 1446890.


2. Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology 2004;126:1175-1189. 15057756.


3. Sarin SK, Sachdev G, Nanda R, Misra SP, Broor SL. Endoscopic sclerotherapy in the treatment of gastric varices. Br J Surg 1988;75:747-750. 3262398.


4. Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, et al. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology 1997;112:889-898. 9041251.


5. Idezuki Y. Present status of sclerotherapy and surgical treatment for esophageal varices in Japan. Japanese Research Society for Portal Hypertension and Japanese Research Society for Sclerotherapy of Esophageal Varices. World J Surg 1992;16:1193-1200. 1455894.


6. Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 1996;11:51-58. 8672742.


7. Kumamoto M, Toyonaga A, Inoue H, Miyakoda K, Morita Y, Emori K, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol 2010;25:1129-1135. 20594229.


8. Akahoshi T, Hashizume M, Tomikawa M, Kawanaka H, Yamaguchi S, Konishi K, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol 2008;23:1702-1709. 18713295.


9. Hiraga N, Aikata H, Takaki S, Kodama H, Shirakawa H, Imamura M, et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol 2007;42:663-672. 17701130.


10. Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol 2005;184:1340-1346. 15788621.


11. Miyamoto Y, Oho K, Kumamoto M, Toyonaga A, Sata M. Balloon-occluded retrograde transvenous obliteration improves liver function in patients with cirrhosis and portal hypertension. J Gastroenterol Hepatol 2003;18:934-942. 12859723.


12. Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol 2001;12:327-336. 11287510.


13. Kawanaka H, Ohta M, Hashizume M, Tomikawa M, Higashi H, Kishihara F, et al. Portosystemic encephalopathy treated with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol 1995;90:508-510. 7872302.

14. Cho SK, Shin SW, Lee IH, Do YS, Choo SW, Park KB, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol 2007;189:W365-W372. 18029851.


15. Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol 2003;4:109-116. 12845306.



16. Choi YS, Lee JH, Sinn DH, Song YB, Gwak GY, Choi MS, et al. Effect of balloon-occluded retrograde transvenous obliteration on the natural history of coexisting esophageal varices. J Clin Gastroenterol 2008;42:974-979. 18528292.


17. Park SJ, Chung JW, Kim HC, Jae HJ, Park JH. The prevalence, risk factors, and clinical outcome of balloon rupture in balloon-occluded retrograde transvenous obliteration of gastric varices. J Vasc Interv Radiol 2010;21:503-507. 20172743.


18. Sarin SK, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol 1989;84:1244-1249. 2679046.


19. Hashizume M, Kitano S, Yamaga H, Koyanagi N, Sugimachi K. Endoscopic classification of gastric varices. Gastrointest Endosc 1990;36:276-280. 2365213.


20. Zoli M, Merkel C, Magalotti D, Marchesini G, Gatta A, Pisi E. Evaluation of a new endoscopic index to predict first bleeding from the upper gastrointestinal tract in patients with cirrhosis. Hepatology 1996;24:1047-1052. 8903373.


21. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Practice Guidelines Committee of the American Association for the Study of Liver Diseases. Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-938. 17879356.


22. de Franchis R. Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-768. 20638742.


23. Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol 2012;18:1-21. 22511898.



24. Akahane T, Iwasaki T, Kobayashi N, Tanabe N, Takahashi N, Gama H, et al. Changes in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol 1997;92:1026-1030. 9177524.

Figure┬Ā1
Rates of variceal eradication or marked shrinkage of all treated gastric varices (GV), gastroesophageal varices (GOV) type 1 (GOV1), GOV type 2 (GOV2), and isolated gastric varices (IGV1).
Figure┬Ā2
Rates of new occurrence and increase in size of esophageal varices (EV). BRTO, balloon-occluded retrograde transvenous obliteration.

Table┬Ā1.
Baseline patient characteristics
Data are presented as mean┬▒SD.
BCLC criteria, Barcelona Clinic Liver Cancer criteria; EV, esophageal varices; GOV1, gastroesophageal varices type 1; GOV2, gastroesophageal varices type 2; GV, gastric varices; IGV1, isolated gastric varices type 1; INR, International Normalized Ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; LC, liver cirrhosis; SD, standard deviations.
Table┬Ā2.
Risk factors for rebleeding and overall survival in 177 patients who completed balloon-occluded retrograde transvenous obliteration (BRTO)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




