Advanced fibrosis is not a negative pretreatment predictive factor for genotype 2 or 3 chronic hepatitis C patients
Article information
Abstract
Background/Aims
Chronic hepatitis C patients with advanced fibrosis have unsatisfactory sustained virological response (SVR) rates. Few data demonstrating the efficacy of combination therapy in chronic hepatitis C patients with advanced fibrosis in South Korea are available. The purpose of this study was to assess whether the stage of fibrosis impacts the efficacy of combination therapy for chronic hepatitis C.
Methods
We retrospectively reviewed data for a total of 109 patients with chronic hepatitis C, treated with peginterferon alfa and ribavirin. SVR according to the stage of liver fibrosis assessed by pretreatment liver biopsy and genotype results were analyzed.
Results
Data from 66 genotype 1 patients (60.6%) and 43 genotype 2 or 3 patients (39.4%) among the 109 patients were analyzed. SVR rates for the genotype 1 patients were significantly lower for the stage 3-4 group (32.1%) than the stage 0-2 group (78.9%; P<0.001). SVR rates (92.0% for stage 0-2, 77.8% for stage 3-4, P=0.184) of genotype 2 or 3 patients were not significantly different according to fibrosis stage. Likewise, the frequency of adverse events was not significantly different according to fibrosis stage.
Conclusions
Compared to patients without advanced fibrosis, we can anticipate good SVR rates for genotype 2 or 3 patients with advanced fibrosis and they did not show an inferior tolerability for peginterferon and ribavirin combination therpy. Our results suggest that active treatment is needed for genotype 2 or 3 patients with advanced fibrosis.
INTRODUCTION
More than 170 million people are infected with hepatitis C virus (HCV) worldwide.1 Approximately 25% of all cirrhosis and hepatocellular carcinoma cases are attributable to chronic hepatitis C infection.2 The prognosis of HCV infection varies according to fibrosis progression, and patients most in need of treatment are chronic hepatitis C with advanced fibrosis or cirrhosis.3
Combination therapy with peginterferon and ribavirin is the optimal treatment for chronic hepatitis C.4 However, antiviral treatment of chronic hepatitis C with advanced fibrosis or cirrhosis is challenging because of frequent comorbidities that affect patient adherence to the scheduled therapies, the risk of adverse events related to therapy, and hyporesponsiveness to peginterferon due to still poorly identified mechanisms.5 Chronic hepatitis C patients with cirrhosis have unsatisfactory sustained virological response (SVR) rates.6 Achieving acceptable SVR rates in these patients significantly reduces the incidence of long-term complications such as hepatic decompensation, development of hepatocellular carcinoma, and death.7 Active treatment of chronic hepatitis C patients with advanced fibrosis is thus very important for preventing such complications.8 However, few data are available on the efficacy of combination therapy in chronic hepatitis C patients with advanced fibrosis in South Korea.6,9
The purpose of this study was to assess whether the stage of fibrosis impacts the efficacy of combination therapy for treating chronic hepatitis C.
PATIENTS AND METHODS
We retrospectively reviewed data from patients with biopsy-confirmed cases of chronic hepatitis C who visited Kyungpook National University Hospital in Daegu, South Korea from March 2004 to December 2007. Inclusion criteria were positivity for HCV antibodies and RNA and liver biopsy results consistent with a diagnosis of chronic hepatitis C. Patients with biopsy-confirmed cases of cirrhosis who had compensated liver disease (in other words, Child-Pugh class A) were also included in the study. Exclusion criteria were history of decompensated liver cirrhosis (ascites, variceal bleeding, jaundice and hepatic encephalopathy), hepatocellular carcinoma, hepatitis B surface antigen (HBs Ag) positivity, severe depression and severe cardiopulmonary disease not controlled by medication.
HCV-RNA was analyzed quantitatively and qualitatively using an Amplicor HCV amplification kit (version 2.0, Roche Diagnostic Systems, Branchburg, NJ, USA). HCV genotyping was performed by a Linear array HCV genotyping test (Roche Molecular Diagnostics, IN, USA). Patients with HCV genotype 1 received either peginterferon alpha-2a (40 kd; Pegasys, Hoffmann-La Roche, Nutley, NJ, USA) 180 µg subcutaneously once weekly regardless of their weight or peginterferon alpha-2b (12 kd; Peg-Intron, Schering-Plough Corporation, Kenilworth, NJ, USA) 1.5 µg/kg subcutaneously once weekly plus ribavirin 1,000 mg perorally daily for patients weighing <75 kg or 1,200 mg perorally daily for ones weighing ≥75 kg (in two separate doses) for 48 weeks. Patients with HCV genotype 2 or 3 received either peginterferon alpha-2a 180 µg subcutaneously once weekly regardless of weight or peginterferon alfa-2b 1.5 µg/kg subcutaneously once weekly plus ribavirin 800 mg perorally daily regardless of weight (in two separate doses) for 24 weeks. The dose of peginterferon was reduced if the absolute neutrophil count (ANC) declined to 750/mm3 or the platelet count declined to 50,000/mm3. Peginterferon administration was stopped if the ANC decreased to 500/mm3 or the platelet count fell to 30,000/mm3. The ribavirin doses was lowered if the hemoglobin concentration decreased to 10.0 g/dL and treatment with ribavirin was stopped if the hemoglobin level declined to 8.0 g/dL.
Early viral response (EVR) was defined as at least a 2-log reduction of HCV RNA after 12 weeks of therapy. EVR was checked for patients infected with genotype 1. End of treatment response (ETR) was defined as the absence of detectable HCV-RNA at the end of therapy. SVR was defined as the absence of serum HCV-RNA at the end of therapy and the following 24 weeks. Adverse events were assessed by clinical evaluation and laboratory tests at regular intervals.
Histologic stage of chronic hepatitis C infection in pretreated liver biopsy specimens was determined based on the scales for scoring fibrosis and cirrhosis of the Scheuer system.10 Fibrosis was recorded as stage 0 (no fibrosis), 1 (portal fibrosis), 2 (periportal fibrosis), 3 (bridging fibrosis or fibrosis with architectural distortion) or 4 (cirrhosis). Patients with stage 3 or 4 are collectively referred to as having advanced fibrosis.11 To compare patients with and without advanced fibrosis, we divided patients into two groups (a stage 0-2 group and stage 3-4 group) according to liver fibrosis severity.
Data were analyzed with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as the mean ± standard error, and the differences between the continuous variables were analyzed by t-tests. Factors influencing the SVR and association between HCV genotype and ETR or SVR were analyzed using a logistic regression model. P-values less than 0.05 were considered statistically significant.
RESULTS
A total of 109 patients were enrolled in this study. Among individuals with genotype 1, 38 (57.6%) were in the stage 0-2 group and 28 (42.4%) were in the stage 3-4 group. Among genotype 2 or 3 patients, 25 (58.1%) were in the stage 0-2 group and 18 (41.9%) were stage 3-4 group. Out of all 109 patients, 91 (83.5%) completed the treatment with peginterferon and ribavirin, while treatment was stopped for 18 patients (Fig. 1). The causes of treatment discontinuation for HCV genotype 1 patients were adverse events (10 patients, 90.9%) and hepatic dysfunction (one patient, 9.1%). The causes of treatment discontinuation for HCV genotype 2 or 3 patients was occurrence of adverse events (three patients, 100%).
The gender distribution, body weight, body mass index (BMI), serum ALT, neutrophil count, and baseline HCV RNA levels for both stage groups (stage 0-2 and stage 3-4) of genotype 1 and genotype 2 or 3 patients were similar while differences in age, serum albumin levels, and platelet counts were observed. Age was significantly higher in the stage 3-4 group for genotype 1 and genotype 2 or 3 patients. Serum albumin levels and platelet counts were significantly lower for the stage 3-4 group among genotype 1 and genotype 2 or 3 patients (Table 1). For genotype 1 individuals, 40 (60.6%) received peginterferon alpha-2a and 26 (39.4%) received peginterferon alpha-2b. For genotype 2 or 3 patients, 26 (60.5%) received peginterferon alpha-2a and 17 (39.5%) received peginterferon alpha-2b.
In the intention-to-treat analysis, ETR rates for genotype 1 patients were significantly lower for the stage 3-4 group (60.7%) compared to the stage 0-2 group (86.8%) (P=0.014; Fig. 2). SVR rates were also significantly lower for the stage 3-4 group (32.1%) than the stage 0-2 group (78.9%; P<0.001).
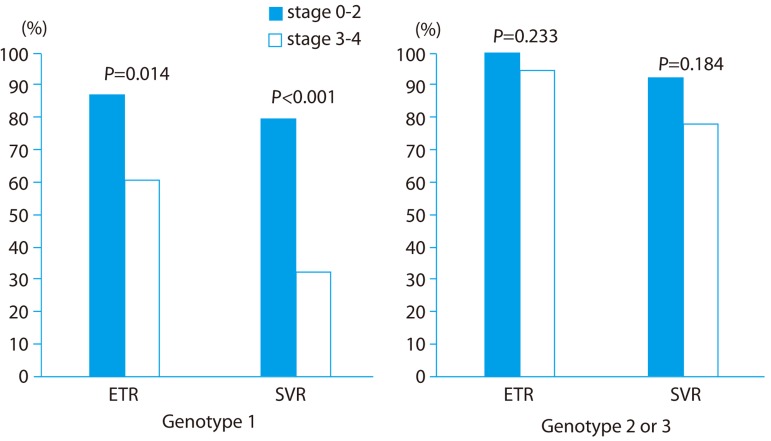
End of treatment response (ETR) and sustained virological response (SVR) rates according to fibrosis stage for genotype 1 and genotype 2 or 3 patients. EVR, early virologic response; SVR, sustained virological response.
No significant differences in ETR (100% for stage 0-2, 94.4% for stage 3-4, P=0.233) or SVR (92.0% for stage 0-2, 77.8% for stage 3-4, P=0.184) rates were observed among genotype 2 or 3 patients according to fibrosis stage.
In the univariate analysis of all of the chronic hepatitis C patients, genotype 1 and fibrosis stage 3-4 were associated with low SVR rates (P=0.004 and P<0.001, respectively; Table 2). In the multivariate analysis, genotype 1 (P=0.033) and peginterferon dose reduction (P=0.022) were significant independent factors associated with low SVR rates (Table 2).

Factors associated with sustained virologic response (SVR) by univariate and multivariate analysis for all of the chronic hepatitis C patients (n=109)
In the multivariate analysis of genotype 1 patients, fibrosis stage 3-4 (P=0.018) and peginterferon dose reduction (P=0.016) were significant independent factors associated with low SVR rates (Table 3). In the multivariate analysis of genotype 2 or 3 patients, there were no significant independent factors associated with low SVR rates (Table 3).

Independent factors associated with negative sustained virologic response (SVR) rates by multivariate analysis for genotype 1 (n = 66) and genotype 2 or 3 patients (n=43)
There were no significant differences between stage 0-2 and stage 3-4 groups for either genotype 1 or genotype 2 or 3 patients in terms of the incidence of adverse events or discontinued therapy due to adverse events (Table 4). Treatment was stopped for a total of 13 patients due to adverse events. These events included anemia, neutropenia, thrombocytopenia, depression, flu-like symptoms and dyspnea. The other types of adverse events observed were rash, itching, nausea, alopecia, weight loss, anorexia and asthenia. One stage 3 patient that was genotype 1 discontinued treatment due to hepatic dysfunction. The rate of peginterferon and ribavirin dose reduction was not significantly different between stage 0-2 and stage 3-4 groups for either genotype 1 and genotype 2 or 3 patients.
DISCUSSION
A subgroup analysis of a randomized trial in Italy12 was similar to the results of our study. For patients infected with genotype 1 or 4, SVR rates were 44% for ones with no or mild fibrosis, 21% for patients with moderate fibrosis, and 24% for individuals with cirrhosis (P=0.04). For patients with HCV genotypes 2 or 3, SVR rates were not significantly influenced by fibrosis stage, and were 89% (no or mild fibrosis), 88% (moderate fibrosis), and 69% (cirrhosis) in patients treated with peginterferon alpha-2a plus ribavirin (P>0.05) and 83% (no or mild fibrosis), 76% (moderate fibrosis), and 64% (cirrhosis) in patients with peginterferon alpha-2b plus ribavirin (P>0.05).12 This study was a rare example in which Caucasian genotype 2 or 3 patients with advanced fibrosis showed good SVR rates.
However, many western studies showed that SVR rates are significantly lower for chronic hepatitis C patients with advanced fibrosis than those without advanced fibrosis for individuals with either genotype 1 or genotype 2 and 3.13-16 In a study by Marrache et al13 SVR rates of 18% (genotype 1), 61% (genotype 2 or 3), and 33% (genotype 4) were reported for patients with advanced fibrosis after treatment with peginterferon alfa-2b plus ribavirin.13 Helbling et al14 also observed an SVR rate of 32% for genotype 1 or 4 patients and 58% for genotype 2 or 3 (P=0.004) patients among individuals with advanced fibrosis.
There is increasing evidence that Asians appear to have considerably better SVR rates than whites according to HCV genotype and treatment regimen. Hepburn et al. demonstrated that Asians (61%) have significantly higher SVR rates than whites (39%).17
A retrospective analysis conducted in a large multicenter Canadian study showed that SVR occurred in 65% Asian and 45% Caucasian (P=0.0047) patients treated with peginterferon alpha-2a plus ribavirin at a fixed dose of 800 mg/day. The ethnic-associated differences were independent of viral genotype and titer, pharmacological regimen, treatment adherence, BMI, age, and hepatic fibrosis.18
These findings were consistent with relatively high SVR rates among genotype 2 or 3 patients with advanced fibrosis from western countries.
Host genetic polymorphisms of interleukin-28B (IL-28B) might determine the rapid virologic response (RVR) rate, the most important predictor of treatment outcome, for Asian patients with genotype 2 infection.19 Unfortunately, We could not analyze the IL-28B and RVR data due to the limitation of retrospective study.
In a recent study of South Korean patients diagnosed with liver cirrhosis clinically that received peginferferon and ribavirin combination therapy, SVR rates were reported to be 20.8% for genotype 1 patients and 52.6% for genotype 2 or 3 patients.20 In the multivariate analysis, HCV genotype 1 is an independent factor associated with low SVR rates.20 It is generally well-known that chronic hepatitis C patients without cirrhosis have low SVR rates if they have HCV genotype 1 and a high viral load prior to treatment.20 It has also been reported that these factors are associated with low SVR rates in patients with compensated liver cirrhosis.9,20-21 However, these studies showed the factors for low SVR rates were analyzed without distinction of HCV genotype. Analysis of efficacy of chronic hepatitis C treatment and response-related factors according to genotype is important because treatment duration and response rates vary according to genotype. Few studies have compared chronic hepatitis C treatment responses according to fibrosis stage between genotype 1 and genotype 2 or 3 South Korean patients with biopsy-confirmed advanced fibrosis.
In our study, genotype 1 was associated with low SVR rate in the analysis of all chronic hepatitis C patients. SVR rates for genotype 1 patients among individuals with advanced fibrosis were significantly lower than those without advanced fibrosis. Advanced fibsosis for genotype 2 or 3 patients was not associated with low SVR rates.
As the limitation of retrospective study, the proportion of patients with biopsy-confirmed cases among all chronic hepatitis C patients during the study period and the indication of liver biopsy were not similar according to genotype. It might be possible that these selection bias influenced SVR rates for genotype 2 or 3 patients with advanced fibrosis.
In our study, there were 10 patients with genotype 1 (10/66, 15.2%) and three with genotype 2 or 3 (3/43, 7.0%) who discontinued treatment due to side effects of the peginterferon and ribavirin combination therapy. This was similar to the rates (5.8-10.3%) reported in South Korean studies of chronic hepatitis C patients.20-22 Among individuals with genotype 1 and genotype 2 or 3, more patients with advanced fibrosis discontinued treatment due to adverse events than ones without, but this is not statistically significant. For genotype 2 or 3 patients, the number of individuals that received reduced doses of peginterferon or ribavirin was lower compared to genotype 1 patients. These results were generally similar to findings (about 20.0-26.7%) reported in a recent South Korean study of patients who were clinically diagnosed with cirrhosis.20 In our study, the percentage of individuals that received reduced peginterferon doses (46.4%) was higher among genotype 1 patients with advanced fibrosis. However, it is difficult to make direct comparisons and there was a possibility of underestimating the rates of adverse events since this was a retrospective study with a relatively small number of patients.
In conclusion, the present study demonstrates that genotype 2 or 3 patients could achieve high SVR rate even if they have advanced fibrosis, and do not have inferior tolerability to peginterferon and ribavirin combination when compared to patients without advanced fibrosis. Our findings suggest that peginterferon and ribavirin combination treatment should be considered actively for genotype 2 or 3 patients with advanced fibrosis.
Notes
The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
ANC
absolute neutrophil count
BMI
body mass index
ETR
end of treatment response
EVR
early viral response
HBsAg
hepatitis B surface antigen
HCV
hepatitis C virus
IL-28B
interleukin-28B
RVR
rapid virologic response
SVR
sustained virological response


