Clinical features and outcomes of gastric variceal bleeding: retrospective Korean multicenter data
Article information
Abstract
Background/Aims
While gastric variceal bleeding (GVB) is not as prevalent as esophageal variceal bleeding, it is reportedly more serious, with high failure rates of the initial hemostasis (>30%), and has a worse prognosis than esophageal variceal bleeding. However, there is limited information regarding hemostasis and the prognosis for GVB. The aim of this study was to determine retrospectively the clinical outcomes of GVB in a multicenter study in Korea.
Methods
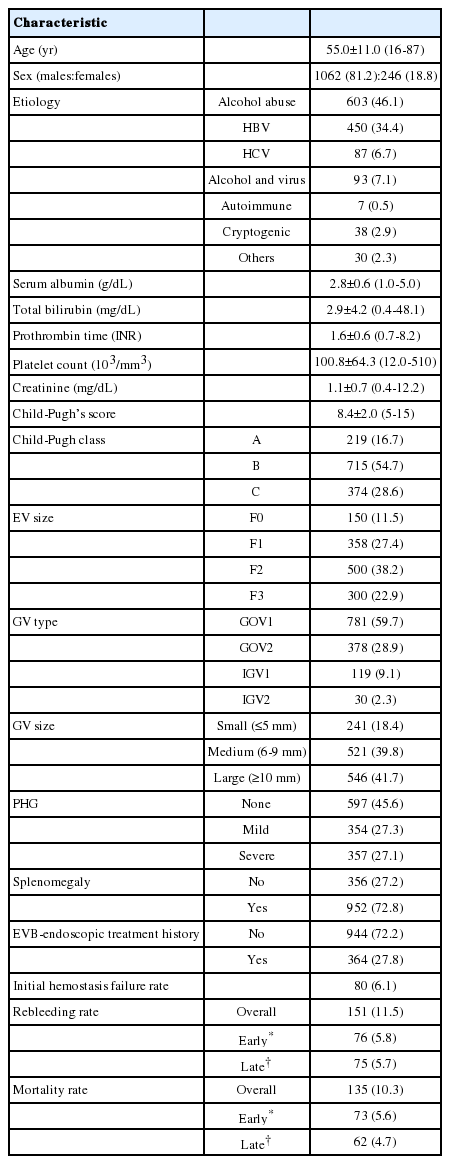
The data of 1,308 episodes of GVB (males:females=1062:246, age=55.0±11.0 years, mean±SD) were collected from 24 referral hospital centers in South Korea between March 2003 and December 2008. The rates of initial hemostasis failure, rebleeding, and mortality within 5 days and 6 weeks of the index bleed were evaluated.
Results
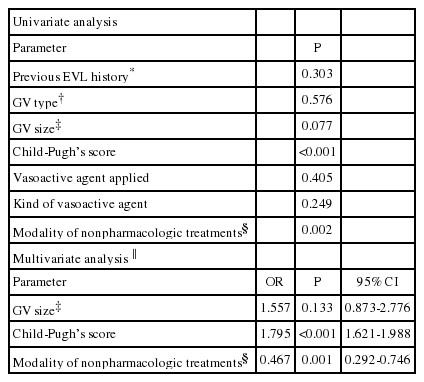
The initial hemostasis failed in 6.1% of the patients, and this was associated with the Child-Pugh score [odds ratio (OR)=1.619; P<0.001] and the treatment modality: endoscopic variceal ligation, endoscopic variceal obturation, and balloon-occluded retrograde transvenous obliteration vs. endoscopic sclerotherapy, transjugular intrahepatic portosystemic shunt, and balloon tamponade (OR=0.221, P<0.001). Rebleeding developed in 11.5% of the patients, and was significantly associated with Child-Pugh score (OR=1.159, P<0.001) and treatment modality (OR=0.619, P=0.026). The GVB-associated mortality was 10.3%; mortality in these cases was associated with Child-Pugh score (OR=1.795, P<0.001) and the treatment modality for the initial hemostasis (OR=0.467, P=0.001).
Conclusions
The clinical outcome for GVB was better for the present cohort than in previous reports. Initial hemostasis failure, rebleeding, and mortality due to GVB were universally associated with the severity of liver cirrhosis.
INTRODUCTION
Gastric varices (GVs) are less common than esophageal varices (EVs), occurring in approximately 20% of patients with portal hypertension.1 However, gastric variceal bleeding (GVB) is reportedly more severe, requires more transfusions, and has a higher mortality rate than the bleeding from EVs.1,2 GVB was hard to control before the 1990s, with relatively high failure rates of the initial hemostasis (>30%) being reported, and the frequency of rebleeding was up to 89% even after the successful hemostasis.3-5 However, since the beginning of this century, the development of various treatment modalities such as endoscopic variceal ligation (EVL), endoscopic variceal obturation (EVO), and balloon-occluded retrograde transvenous obliteration (BRTO) has resulted in higher success rates for the initial control of bleeding (>90%) as well as lower rates of rebleeding (20-30%) than was previously reported.6,7 In addition, the cumulative survival rate of one cohort at 6 years was reported to be 43%. Yet, the recent clinical data for GVB is still limited and rare especially in Korea.
The management of GVs has some inherent difficulties. The inhomogeneity of GVs demands various treatment methods and techniques that vary with the GV type. However, the treatment tends to be empiric, since consensus regarding the optimum treatment for GVs has not been reached yet due to insufficient basic data on GVB.8 Therefore, the aim of this multicenter retrospective study was to elucidate the general characteristics of GVB and the clinical outcomes including rebleeding and mortality, as well as the treatment modalities currently used in Korea.
PATIENTS AND METHODS
Patients and data collection
Participants were drawn from a consecutive series of 1308 cirrhotic patients admitted for hematemesis and/or melena due to GVB (as confirmed by endoscopic evaluation) at 24 medical centers distributed throughout Korea between January 2003 and December 2008. The medical records of the enrolled patients were reviewed to obtain the necessary demographic, clinical, laboratory, treatment, and follow-up data. Patients who were combined with malignancy including hepatocellular carcinoma were excluded. The therapeutic methods of hemostasis for GVB and the development of rebleeding or death related to GVB were also reviewed. The diagnosis of cirrhosis was based on a previous liver biopsy or on compatible clinical, laboratory, and imaging findings. In addition, time intervals from hospital admission to the start of each therapy, such as administration of the vasoactive agent administered or endoscopy, were reviewed. All patients were followed up to the time of death or 42 days after the hospital admission.
In the analysis, the therapeutic modalities were divided into two groups; endoscopic variceal ligation (EVL), endoscopic variceal obturation (EVO) and balloon-occluded retrograde transvenous obliteration (BRTO) vs. endoscopic injection sclerotherapy (EIS) transjugular intrahepatic portosystemic shunt (TIPS), balloon tamponade and no therapy. The reason of this grouping is that the former is generally accepted as standard therapy, on the contrary to this, the latter is considered as salvage or bridge therapy.
The sizes of the EVs were classified according to the criteria of Beppu et al.9 GVs were classified as gastroesophageal varices (GOVs), either associated with EVs along the lesser curvature (GOV1) or along the fundus (GOV2), and isolated gastric varices (IGVs), which are present in isolation in the fundus (IGV1) or at ectopic sites in the stomach or the first part of the duodenum (IGV2), as determined using the criteria of Sarin et al.1 GOV2s and IGV1s were also analyzed as 'fundal varices.' The size of each GV was classified as small (≤5 mm), medium (6-9 mm), or large (≥10 mm). The hepatic function of patients at admission was estimated according to the Child-Pughs score.10,11 The ethics committee of each hospital approved the protocol.
Definitions
The following definitions were based on recommendations from the Baveno II, III, and IV consensus workshops and previous literature: 12-17
1. Time zero of the bleeding episode: the time of admission to the first hospital to which the patient was taken.
2. Acute GVB: a lesion actively bleeding or with an adherent clot, white nipple, or presence of a single lesion on the GVs without other potential sources of bleeding or EVs.
3. Index bleeding: bleeding was defined as active if a spurting or oozing lesion was seen on endoscopy, or if fresh blood was regurgitated via the gastric tube when endoscopy was not allowed.
4. Success of initial hemostasis: the acute bleeding episode was considered finished at the beginning of the first 24-hr bleeding-free interval with no hematemesis, stable hemoglobin concentration without blood transfusions, and stable hemodynamic conditions (absence of systolic blood pressure or a reduction of 20 mmHg or more, and an increase in heart rate of 20 beats/min or more).
5. Rebleeding: any occurrence of hematemesis or hemoglobin drop with fresh melena after initial hemostasis has been achieved.
Treatment outcomes were assessed in accordance with the failure to initial hemostasis of index bleeding, rebleeding, and death within 5 days (early) and 42 days (late) from the index bleeding. For the analysis of mortality, death within 42 days (6 weeks) of GVB was defined as a GVB-related death.12-18
Statistical analysis
The results are expressed as mean±SD values. Categorical variables were analyzed with a Pearson's chi-square test. Noncategorical variables were compared with independent t-tests, ANOVA. A multivariate analysis for factors associated with the failure of initial hemostasis, rebleeding, and mortality were conducted using binary logistic regression analysis. The factors with P<0.1 in univariate analyses were included in multivariate analyses using enter method. All tests were two-tailed, and the level of statistical significance was set at P<0.05. Statistical analyses were performed with SPSS for Windows, version 12.0 (SPSS, Chicago, IL, USA).
RESULTS
Characteristics of the cohorts
The general characteristics of the cohort at admission are summarized in Table 1. The age of the patients was 55.0±11.0 years (range, 16-87 years), and 81.2% (n=1,062) were male. Liver cirrhosis was caused by alcohol in 46.1%, hepatitis B virus in 34.4%, hepatitis C virus in 6.7%, both alcohol and the virus in 7.1%, autoimmune hepatitis in 0.5%, cryptogenic in 2.9%, and others in 2.3% of the cohort. A previous history of endoscopic treatment due to EV bleeding was noted in 364 of the 1308 patients (27.8%). The type of GV was GOV1 in 781 patients (59.7%), GOV2 in 378 patients (28.9%), IGV1 in 119 patients (9.1%), and IGV2 in 30 patients (2.3%).
Initial hemostasis
Pharmacologic treatments using vasoactive agents were applied in 87.6% of the entire cohort as an initial hemostatic method as soon as possible before endoscopic or other interventional therapies were instituted. These vasoactive agents were administered for 70.9±33.5 hours at standard doses. Among the vasoactive agents, terlipressin was the most frequently used (50.3%), followed in order by somatostatin (37.2%), octreotide (11.4%), and vasopressin with or without nitrate (1.1%) (Fig. 1).
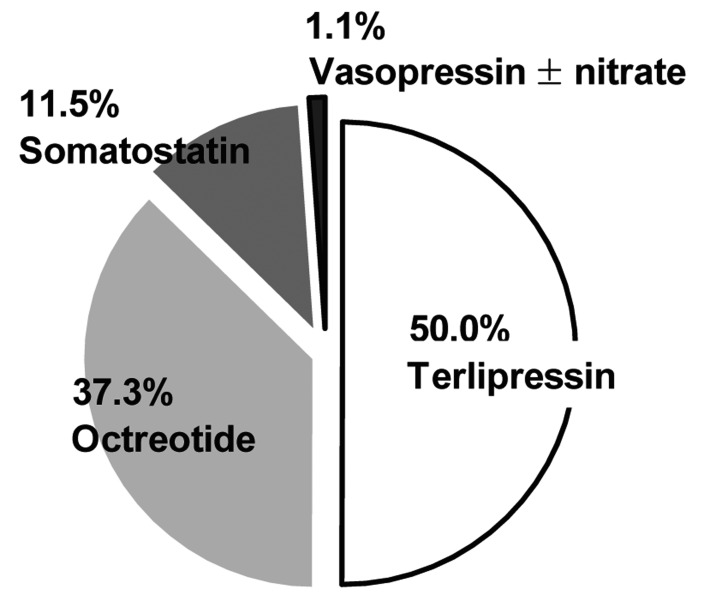
Type of intravenous vasoactive agents applied to treat the initial acute gastric variceal bleeding (GVB) among the entire cohort.
Endoscopic, radiologic, or other interventional treatments were performed in 1,211 of the 1,308 episodes (92.6%) for initial hemostasis or for prevention of a rebleeding of the index bleed. The treatment included endoscopic variceal ligation (EVL; n=553, 42.3%), EVO (n=484, 37.0%), BRTO (n=60, 4.6%), EIS (n=52, 4.0%), TIPS (n=37, 2.8%), and balloon tamponade (n=25, 1.9%). Ninety-seven patients (7.4%) experienced spontaneous hemostasis and did not receive any treatment (Fig. 2).

Type of nonpharmacologic treatment modalities that were applied to control the initial acute GVB. Endoscopic variceal ligation (EVL) and endoscopic variceal obturation (EVO) were the predominantly applied modalities. BRTO, balloon-occluded retrograde transvenous obliteration; EIS, endoscopic injection sclerotherapy; TIPS, transjugular intrahepatic portosystemic shunt; BT, balloon tamponade; No Tx., no treatment.
The failure rate of the initial hemostasis was 6.1% (80/1308 patients). There was no significant association between failure of hemostasis and the type of GV, with failure occurring in 55 (7.1%), 21 (5.5%), 3 (2.5%), and 1 (3.3%) of cases of GOV1, GOV2, IGV1, and IGV2, respectively (P=0.207). In addition, the subanalysis revealed that with the exception of just one case of IGV2, the initial failure rate did not differ between GOV1 (cardiac varix; n=55, 7.1%) and GOV2+IGV1 (fundal varix; n=24, 4.8%; P=0.121). The Child-Pugh score (P<0.001) and the modality of nonpharmacologic treatments for the initial hemostasis were significantly related to the failure of the initial hemostasis. The failure rates for Child-Pugh classes A, B, and C were 0.5%, 3.5%, and 14.4%, respectively (P<0.001). The initial hemostasis failure rate was lower in patients treated with EVL, EVO, and BRTO than in those treated with EIS, TIPS, balloon tamponade and no therapy (P<0.001; Table 2, Fig. 3). However, the initial treatment failure rate of GOV1 bleeding did not differ significantly among EVL, EVO, and BRTO (P=0.466).
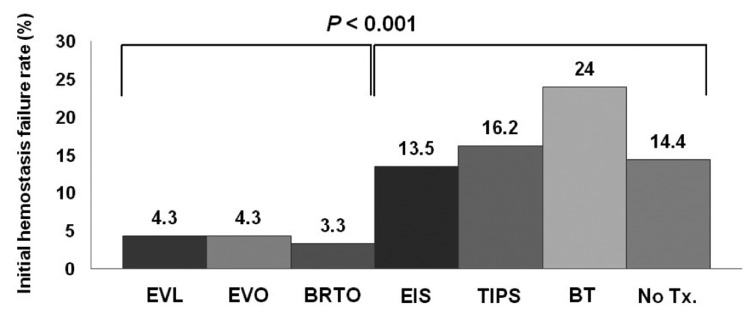
Comparison of initial hemostasis failure rates according to the initial nonpharmacologic treatment modality applied (EVL, EVO, and BRTO vs. the others). The failure rate was significantly lower for EVL, EVO, and BRTO than for the other modalities, and did not differ among EVL, EVO, and BRTO.
The application of vasoactive agents and the type thereof did not affect the outcome of initial hemostasis in the entire cohort (P=0.722 and 0.360, respectively). In multivariate analysis with logistic regression analysis, the Child-Pugh's score (odds ratio [OR]=1.619, 95% confidence interval [CI]=1.437-1.823) and the modality of the initial hemostasis (EVL, EVO, and BRTO vs. the others; OR=0.221, 95% CI=0.131-0.371) were significantly associated with initial hemostasis failure (Table 2).
Rebleeding
The overall rebleeding rate was 11.5% (151/1308 patients). Early rebleeding within 5 days of the index bleeding occurred in 5.8% (76 patients), and late rebleeding from the 6th day to the end of 6 weeks of the index bleeding occurred in 5.7% (75 episodes) of the entire cohort. The rebleeding rate did not differ significantly with the GV type: 10.8% (84/781) of those with GOV1, 12.5% (62/497) of those with fundal varices, and 16.7% (5/30) of those with IGV2 (P=0.240). The application of vasoactive agents and the type thereof did not affect the development of rebleeding in the entire cohort (P=0.598 and 0.869, respectively). However, significant correlations with rebleeding were obtained for previous EVL history (P=0.002), the size of the GV (P=0.032), and Child-Pugh score (P<0.001). The rebleeding rate was 5.0%, 10.9%, and 16.6% for Child-Pugh classes A, B, and C, respectively (P<0.001). In addition, the use of EVL, EVO, or BRTO as an initial hemostasis modality was associated with a significantly lower rebleeding rate (P=0.033). In multivariate analysis, previous EVL history (OR=1.781, 95% CI=1.246-2.546), Child-Pugh's score (OR=1.159, 95% CI=1.068-1.258), and the modality of the initial hemostasis (EVL, EVO, or BRTO; OR=0.619, 95% CI=0.406-0944) were significantly associated with rebleeding (Table 3).
Mortality
The overall mortality caused by GVB was 10.3% (135/1308 patients). Early deaths within 5 days of the index bleeding occurred in 5.6% of the cohort (73/1308 patients), and late deaths during days 6-42 after the index bleeding occurred in 4.7% (62/1308 patients). The mortality did not differ significantly between cases of GOV1 bleeding (11.0%, 86/781) and fundal variceal bleeding (9.9%, 49/497; P=0.576). There were no cases of mortality among those with IGV2 bleeding. The mortality was lower among patients treated with either EVL, EVO, or BRTO than those treated with EIS, TIPS, or balloon tamponade (P=0.002), although the outcome did not differ significantly among the EVL, EVO, and BRTO groups (P=0.625; Fig. 4). There was also a significant relationship between Child-Pugh's score and mortality (P<0.001; Table 4): the mortality rate was 0%, 5.3%, and 25.9% for those with Child-Pugh class A, B, and C, respectively. Multivariate analysis revealed a significant relationship between mortality and the both Child-Pugh's score (OR=1.795, 95% CI=1.621-1.988) and modality of the initial hemostasis (EVL, EVO, BRTO vs. the others; OR=0.467, 95% CI= 0.292-0.746; Table 4).
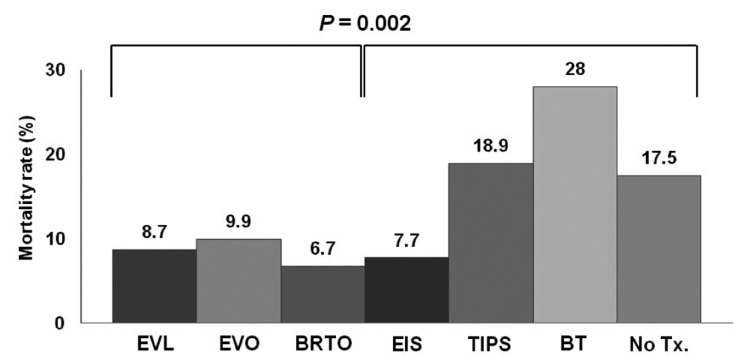
Comparison of mortality rates according to the initial nonpharmacologic treatment modality applied (EVL, EVO, and BRTO vs. the others). The mortality rate was significantly lower for EVL, EVO, and BRTO than for the other modalities, and did not differ significantly between EVL, EVO, and BRTO.
DISCUSSION
While the knowledge and treatment modalities for GVs have improved, little remains known about the general characteristics of GVs and GVB. We thus dependent upon the data from two decades ago.1 In Korea, one study reported the cumulative incidence rates of GVB as 4.8%, 19.9% and 23.2% at 1, 3 and 5 years after diagnosis and overall survival rates in patients with gastric varices were 88.6%, 53.2% and 37.2% at 1, 5 and 10 years respectively.17 However, little has been known about the clinical outcome of GVB in Korea. The study presented here analyzed a consecutive series of 1,308 cases of GVB that occurred in patients with liver cirrhosis during the past 6 years, collected from 24 medical centers distributed throughout South Korea. Thus, despite being performed retrospectively, this study can provide recent, representative information regarding the clinical features and treatment outcomes of GVB in this country.
The natural history of GVB differs from that of EV bleeding. Although the risk of bleeding from a GV is lower than that from an EV, once bleeding has occurred, the transfusion requirements and the risk of mortality are greater for GVB.1,18 The mortality from the first GVB was reported as high as 20% within 6 weeks of the index bleeding and among the type of GV, GOV2s frequently bled and were associated with high mortality.1-3 However, in the present study, the overall mortality rate was half of that, at 10.3%, and it did not differ between cardiac and fundal varices. This reduction in mortality may be at least partly attributable to the reduction in the initial hemostasis failure and rebleeding rates due to advancements in endoscopic and radiologic interventional treatments, as well as to some severe cases possibly having been dead upon their arrival at the hospital (and thus not included in our cohort). Of note, even though the success rate of the initial hemostasis was similar to that reported recently, the rebleeding rate in the present study (11.5%) was much lower than that reported in recent studies (20-30%).6,7,19,20
There are various therapeutic options available for the treatment of GVB, including EVL, EIS, EVO, TIPS, BRTO, and balloon tamponade. EVO has emerged as a particularly effective initial treatment for GVB, and especially in cases of fundal varices.5,21,22 In the present study, EVL, EVO, and BRTO were associated with a lower initial hemostasis failure, rebleeding, and mortality rate compared to the other therapeutic modalities. In addition, no significant difference was observed regarding these clinical outcomes between EVL and EVO in cases of GOV1. However, these findings should be interpreted with caution because of the retrospective design of this study and the uneven distribution of cases among the different types of modality. Furthermore, EIS or balloon tamponade might have been applied in worse clinical situations and levels of preparation compared with EVL or EVO. A large-scale prospective trial is required to confirm these data. In addition, although BRTO, TIPS and balloon tamponade are generally not considered as initial therapy of GVB, in Korea as we showed in this study, they have been applied as the primary hemostatic method for GVB in some centers. Unexpectedly, some clinicians hesitated to apply prompt EVO because of its invasiveness and potential risk of complication and they preferred to apply BRTO or TIPS instead whenever if it is possible (data was not shown).
In general, it is known that EVL is associated with higher hemostasis failure and rebleeding rates in GVB compared to EVO, since GVs tend to be larger and deeper than EVs.19,20 EVL clinical data stratified according to GV type are lacking. In the present study, the control of bleeds from GOV1s (which are linked with EVs) using EVL yielded similarly good results with regard to initial hemostasis and mortality compared to EVO, irrespective of the GV size. This result may have been influenced by advancements in endoscopic equipment and the EVL technique. Therefore, bleeding from a GOV1 just below the gastroesophageal junction would be a good candidate for management with EVL.
GVs have a high rebleeding rate (20-90%), especially in cases of fundal varices.3-5,23 This may be attributable to incomplete obliteration and mucosal injury, respectively.4,24 Larger fundal varices with greater blood flows may be responsible for the low eradication rates. However, these data are based on the old studies in which sclerotherapy employed tetradecyl sulfate, alcohol, or bucrylate.25 Data regarding rebleeding or mortality for the therapeutic modalities that have recently been developed are very limited. In particular, there are few data related to the GV type. In the present study, the overall GV rebleeding rate was just 11.5%, with no difference between cardiac (GOV1) and fundal varices. Advances in endoscopic and radiologic interventions may be responsible for this decrease in the rebleeding rate relative to other studies. Technical improvements in the EVL with multiband equipment and EVO using N-butyl-2-cyanoacrylate (Histoacryl) may have been responsible for the remarkable improvement in the clinical outcome of GVB to levels similar to that of EVs.26
Finally, among the many related factors, the Child-Pugh's score was significantly associated with all clinical outcomes, including initial hemostasis failure, rebleeding, and mortality, irrespective of the GV type or size. This suggests that the only important factor in predicting the prognosis of GVB is the severity of cirrhosis. However, since this study was conducted retrospectively, this limitation has to be considered in the interpretation of results, and well designed prospective studies are needed.
In summary, we have shown that the treatment outcome of GVB has improved dramatically in recent years, and that the most important factor determining the clinical outcome of GVB is the severity of cirrhosis. Not only were initial hemostasis and rebleeding strongly associated with Child-Pugh's score, but so also was mortality, regardless of the GV type and size.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea. (A102065).
Notes
The authors have no conflicts to disclose.
Abbreviations
EV
esophageal varix
GV
gastric varix
GOV1
gastro-oesphageal varix type 1
GOV2
gastro-oesphageal varix type 2
IGV1
isolated gastric varic type 1
IGV2
isolated gastric varic type 2
EVB
esophageal variceal bleeding
GVB
gastric variceal bleeding
EVL
endoscopic variceal ligation
EVO
endoscopic vascular obturation
BRTO
balloonoccluded retrograde transvenous obliteration
EIS
endoscopic injection sclerotherapy
TIPS
transjugular intrahepatic portosystemic shunt
BT
balloon tamponade
HBV
hepatitis B virus
HCV
hepatitis C virus
INR
international normalized ratio
PHG
portal hypertensive gastropathy
OR
odds ratio
CI
confidence interval