The usefulness of non-invasive liver stiffness measurements in predicting clinically significant portal hypertension in cirrhotic patients: Korean data
Article information
Abstract
Background/Aims
Liver stiffness measurement (LSM) has been proposed as a non-invasive method for estimating the severity of fibrosis and the complications of cirrhosis. Measurement of the hepatic venous pressure gradient (HVPG) is the gold standard for assessing the presence of portal hypertension, but its invasiveness limits its clinical application. In this study we evaluated the relationship between LSM and HVPG, and the predictive value of LSM for clinically significant portal hypertension (CSPH) and severe portal hypertension in cirrhosis.
Methods
LSM was performed with transient elastography in 59 consecutive cirrhotic patients who underwent hemodynamic HVPG investigations. CSPH and severe portal hypertension were defined as HVPG ≥10 and ≥12 mmHg, respectively. Linear regression analysis was performed to evaluate the relationship between LSM and HVPG. Diagnostic values were analyzed based on receiver operating characteristic (ROC) curves.
Results
A strong positive correlation between LSM and HVPG was observed in the overall population (r2=0.496, P<0.0001). The area under the ROC curve (AUROC) for the prediction of CSPH (HVPG ≥10 mmHg) was 0.851, and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for an LSM cutoff value of 21.95 kPa were 82.5%, 73.7%, 86.8%, and 66.7%, respectively. The AUROC at prediction of severe portal hypertension (HVPG ≥12 mmHg) was 0.877, and the sensitivity, specificity, PPV, and NPV at LSM cutoff value of 24.25 kPa were 82.9%, 70.8%, 80.6%, and 73.9%, respectively.
Conclusions
LSM exhibited a significant correlation with HVPG in patients with cirrhosis. LSM could be a non-invasive method for predicting CSPH and severe portal hypertension in Korean patients with liver cirrhosis.
INTRODUCTION
The prognosis and management of chronic liver diseases strongly depend on the severity of portal hypertension.1-6 Measurement of the hepatic venous pressure gradient (HVPG) is the gold standard for the assessment of the presence of portal hypertension and correlates with the occurrence of its complications in patients with cirrhosis. However, HVPG measurements are invasive, relatively expensive and available only in specialized centers with well-trained operators. Transient elastography (TE, Fibroscan®, Echosens, France) is a method that allows the measurement of liver stiffness. TE can be performed in the outpatient clinic with immediate results and excellent reproducibility. TE has been proposed as a non-invasive method for the prediction of the severity of fibrosis and the complication of cirrhosis.7-10 An liver stiffness measurement (LSM) value above 14.6 kPa diagnosed cirrhosis with 0.79 sensitivity and 0.95 specificity in a large cohort of patients with various causes of chronic liver diseases.11 Several studies have demonstrated that TE may represent a rapid and noninvasive method for predicting the presence of clinically significant (HVPG ≥10 mmHg) or severe (HVPG ≥12 mmHg) portal hypertension.12-15 However, LSM exhibited a relatively weak correlation with HVPG ≥12 mmHg, likely because of the increasing relevance of extrahepatic factors in the progression of portal hypertension such as increases in portal blood flow.13 However, a similar study of Korean data are lacking. In this study, we evaluated the relationship between LSM and HVPG, and the predictive value of LSM for clinical significant portal hypertension and severe portal hypertension in cirrhosis.
PATIENTS AND METHODS
Patients
Fifty-nine consecutive patients with cirrhosis (48 men and 11 women, mean age 49.5±8.9; age range 29-69 years) underwent HVPG measurement between February 1, 2009 and February 1, 2010 upon referral to the hemodynamic laboratory of our institution. All patients had previous histopathological confirmation of cirrhosis (METAVIR F4)16 or a diagnosis of cirrhosis suspected on the basis of standard clinical, ultrasonographic, and biochemical parameters. Exclusion criteria were an age younger than 19 or older than 75 years, shock status requiring a vasopressor, active infection (e.g., spontaneous bacterial peritonitis), acute renal failure of any cause, hepatocellular carcinoma or a history of another primary malignancy within 3 years, clinically relevant coronary artery disease (NYHA functional angina classification III/IV), congestive heart failure NYHA III/IV, clinically relevant cardiomyopathy, history of myocardial infarction in the past 1 year, poorly controlled hypertension (blood pressure >150/100) mmHg, transaminases above tenfold the upper limit of normal, ongoing antiviral therapy, the use of vasoactive drugs, including beta-blockers, diuretics, and anti-inflammatory drugs, pregnancy or lactation, involvement in another study within 90 days, and medical or psychological conditions that would not permit the subject to complete the study or sign informed consent. The study protocol was approved by the Institutional Review Board for Human Research at the Yonsei University Wonju Severance Christian Hospital (Wonju, Republic of Korea). The nature of the study was explained to all patients, each of whom provided written informed consent before the beginning of the study, in accordance with the principles of the Declaration of Helsinki (revision of Edinburgh, 2000).
Transient elastography
After an overnight fasting, all patients underwent a complete abdominal ultrasonography examination in the morning. Immediately following the examination, TE was performed by two experienced operators. The Fibroscan device (Fibroscan®, Echosens, France) consists of a 3.5 MHz ultrasound transducer M probe mounted on the axis of a vibrator. Mild amplitude and low-frequency vibrations (50 Hz) are transmitted to the liver tissue, inducing an elastic shear wave that propagates through the underlying liver tissue. TE was performed as previously described on the right lobe of the liver; in the intercostal space with the patient lying in dorsal decubitus with the right arm at maximal abduction.17-18 The operator, assisted by an ultrasonic time-motion image, located a liver portion at least 6 cm thick and free of large vascular structures and the gallbladder. Ten successful measurements were performed on each patient. The success rate was calculated as the ratio of the number of successful measurements over the total number of acquisitions. Only LSM with an interquartile range ≤30% of the median value and a success rate of at least 60% were considered reliable. Results are expressed in kilopascals (kPa) and the median value was used a representative of LSM.
Measurements of HVPG
After an overnight fast, the HVPG was measured. The right hepatic vein was catheterized percutaneously through the femoral vein, and the pressures in both the wedged and the free positions were recorded with a 7-F balloon-tipped catheter (Arrow Deutschland, PostfachErding, Germany). In case that shunt was observed in right hepatic vein, HVPG was measured in middle hepatic vein. All measurements were performed at least in triplicate, and permanent tracings were obtained on a multichannel recorder.19,20 HVPG was determined by subtracting the free hepatic venous pressure from the wedged hepatic venous pressure.3 An examiner (Y.J.K.) with 12 years of experience with HVPG measurement performed all HVPG procedures. Clinically significant portal hypertension (CSPH) was defined as an HVPG ≥10 mmHg according a consensus definition.21 Severe portal hypertension was defined as an HVPG ≥12 mmHg.
Laboratory parameters
Laboratory Parameters were obtained in all patients on the day of HVPG measurement and included serum albumin, bilirubin, alanine aminotransferase, aspartate aminotransferase, platelet count, hemoglobin, hematocrit, white blood cell, International Normalized Ratio (INR), blood urea nitrogen, creatinine, electrolyte, and C-reactive protein. The model for end-stage liver disease (MELD) and the Child-Pugh score were calculated.
Statistical analysis
Statistical analyses performed using SPSS 18.0 for Windows. All results are expressed as mean±standard deviation (SD). Linear regression analyses were calculated according to the least squares methods. P-values less than 0.05 were considered statistically significant. The statistical significance of inter-group differences was evaluated by the means of the spearman rank correlation coefficient. The relationship between sensitivity and specificity of LSM at different cutoff points; as a predictor of patients with HVPG ≥10 mmHg or patients with HVPG ≥12 mmHg was evaluated by receiver operating characteristic (ROC) curves. Optimal liver stiffness cutoff values were selected on the basis of sensitivity, specificity, and positive and negative predictive values.
RESULTS
General characteristics of the patients
The characteristics of the patients are summarized in Table 1. The etiology of cirrhosis was classified as alcohol (36, 61.0%), hepatitis B virus (13, 22.0%), alcohol with hepatitis B virus (5, 8.5%), alcohol with hepatitis C virus (2, 3.4%), and cryptogenic cirrhosis (3, 5.1%). The mean Child-Pugh and MELD scores were 6.5±1.8 and 9.6±3.7 (range 6-21), respectively. Mean HVPG and LSM were 13.1±5.2 mmHg (range 5-24) and 36.5±23.6 kPa (range 5.4-75.0), respectively.
Relationship between HVPG and LSM
Considering the whole patient population, a statistically significant positive correlation between HVPG and LSM was found (Fig. 1). Figure 1A demonstrates significant linear correlation between HVPG and LSM (r2=0.496, P< 0.05). In patients with HVPG ≥10 mmHg or ≥12 mmHg, there was a statistically significant correlation with LSM (r2=0.297, P<0.05 and r2=0.192, P=0.05, respectively) (Fig. 1B and 1C). However, in case of HVPG ≥12 mmHg, the correlation was relatively weak compared with that of the HVPG ≥10 mmHg group.
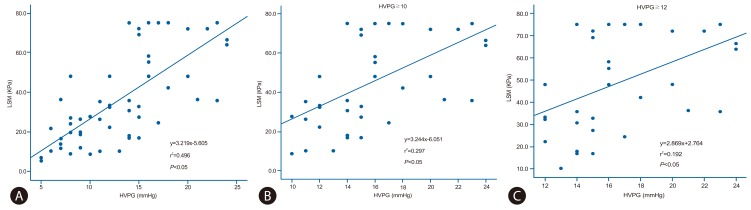
Analysis of the linear regression between the hepatic venous pressure gradient (HVPG) and liver stiffness measurement (LSM). A strong positive correlation between LSM and HVPG was observed in the overall population (r2=0.496, P<0.05) (A) and in the subgroups with HVPG ≥10 mmHg (r2=0.297, P<0.05) (B) and HVPG ≥12 mmHg (r2=0.192, P<0.05) (C).
Diagnostic value of LSM for the non-invasive prediction of clinically significant portal hypertension
Figure 2 presents the ROC curve of LSM for the prediction of CSPH. In case of HVPG ≥10 mmHg, the area under the ROC curves (AUROC) was 0.851 (95% confidence index 0.752-0.949). In cases of HVPG ≥12 mmHg, the AUROC was 0.877 (95% confidence index 0.792-0.962). Based on the ROC curves, different cutoff values for LSM were determined. An LSM ≥21.95 kPa exhibited a positive predictive value (PPV) of 86.8% and a negative predictive value (NPV) of 66.7% with a sensitivity of 82.5% and a specificity 73.7% for the prediction of patients with HVPG ≥10 mmHg. An LSM ≥24.25 kPa exhibited a PPV of 80.6% and an NPV of 73.9% with a sensitivity of 82.9% and a specificity of 70.8% for the prediction of patients with HVPG ≥12 mmHg.
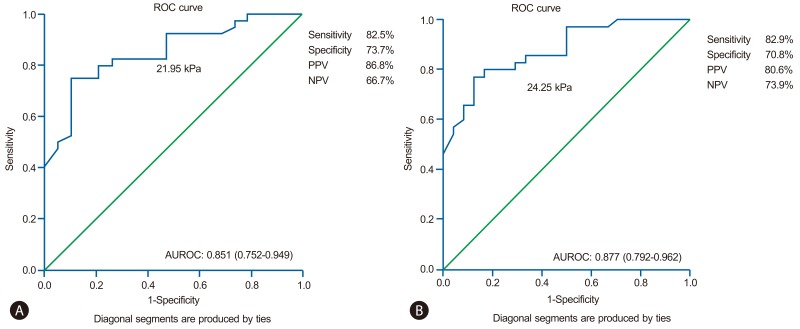
Receiver operating characteristic (ROC) curves demonstrating the prediction of clinically significant portal hypertension (i.e., HVPG ≥10 mmHg) (A) and severe portal hypertension (i.e., HVPG ≥12 mmHg) (B) with transient elastography in the entire patient population; the corresponding areas under the ROC curves (AUROC) were 0.851 and 0.877, respectively. PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
The prognosis and management of chronic liver diseases strongly depend on the severity of portal hypertension. Therefore, the assessment of portal hypertension is one of the most important steps in the management of chronic liver disease. The best method to diagnose portal hypertension is the direct measurement of portal pressure. HVPG is currently the gold standard for the diagnosis of intrahepatic portal hypertension. In healthy individuals, HVPG is below 5 mmHg. An HVPG greater than 5 mmHg is suggestive of the presence of portal hypertension, but the complications related to portal hypertension, such as asicites or variceal bleeding, tend to appear if HVPG ≥10 mmHg,22,23 therefore this threshold is known as clinically significant portal hypertension.
Although HVPG measurement is a safe procedure, it is considered as a relatively invasive method. Therefore the development of new non-invasive markers of portal hypertension is encouraged.24,25 From the initial report of the new technique to quantify the amount of liver fibrosis by LSM using TE,18 many studies have validated this method in the diagnosis of liver fibrosis and cirrhosis. A few studies demonstrated the good correlation between liver stiffness and HVPG.12-15,26 In these studies, LSM can diagnose clinically significant portal hypertension with an AUROC varying between 0.76 and 0.99. However, the problem is that the cut-off value for clinically significant portal hypertension is variable, between 13.6 kPa and 34.9 kPa. These differences may be due to the heterogeneity of the studied population and different etiologies. Liver stiffness is more elevated in alcoholic liver disease compared to viral liver disease.15 The optimal cut-off values are higher in the alcoholic group than in the hepatitis C virus infected group (34.9 kPa vs. 20.5 kPa).15 It suggests that the LSM values must be closely interpreted according to the cause of the liver disease.
Furthermore, liver stiffness is very well correlated with HVPG up to values of 10-12 mmHg, but above these values the correlation is lower, demonstrating that portal hypertension is only partially caused by the amount of fibrosis.27
In our study, the main result is that LSM is strongly correlated with HVPG and accurately predicts the presence of clinically significant portal hypertension in patients with cirrhosis in a Korean population. Interestingly, in patients with HVPG ≥10 mmHg or ≥12 mmHg, there was a statistically significant correlation with LSM (r2=0.297, P<0.0001 and r2=0.192, P=0.009, respectively). With a cutoff of LSM ≥21.95 kPa, the NPV and PPV for the diagnosis of CSPH (HVPG ≥10 mmHg) were 66.7% and 82.5%, respectively. Moreover, the presence of severe portal hypertension (HVPG ≥12 mmHg) could be suspected when a cutoff value ≥24.25 kPa was reached, with an NPV and PPV of 73.9% and 82.9%, respectively. In our study, the cutoff of LSM for the diagnosis of CSPH was different different from previous reports indicating cutoff values of 12.5 kPa, 13.6 kPa, and 14.5 kPa.7,8,12,13 Because most of our study population exhibited alcoholic cirrhosis, the cutoff values may higher than previous reports.7,8,12,13 As mentioned previously, liver stiffness is more elevated in alcoholic liver disease compared with viral liver disease.15
The major limitations of this study are the small sample size and the fact that the cirrhosis has not been confirmed by liver biopsy in all the patients. However, this is the first Korean study of a relationship between LSM and HVPG. Based on this study, further carefully designed studies will be conducted in the future.
In summary, LSM exhibited significant correlation with HVPG in patients with cirrhosis. LSM could be a non-invasive method to predict clinically significant and severe portal hypertension in patients with liver cirrhosis in Korea.
Acknowledgments
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, (HI10C2020).
Notes
The authors have no conflicts to disclose.
Abbreviations
AUROC
areas under ROC curves
CSPH
Clinically significant portal hypertension
HVPG
hepatic venous pressure gradient
LSM
liver stiffness measurement
NPV
negative predictive value
PPV
positive predictive value
TE
transient elastography
