Two cases of telbivudine-induced myopathy in siblings with chronic hepatitis B
Article information
Abstract
Telbivudine is an L-nucleoside analogue with potent antiviral activity against hepatitis B virus (HBV). Clinical trials have shown that telbivudine has a more potent and sustained antiviral activity with a lower frequency of viral resistance than lamivudine. Although there are several reports concerning the safety profile of telbivudine, most adverse events are described as mild and transient in nature. Here we report two cases of telbivudine-induced myopathy in patients with chronic hepatitis B who were siblings.
INTRODUCTION
Chronic hepatitis B (CHB) is a major health problem worldwide. It is estimated that approximately 400 million people are infected with hepatitis B virus (HBV) globally.1 CHB can progress to cirrhosis, decompensated cirrhosis, hepatocellular carcinoma (HCC), and death.2 Korea is an HBV endemic area;2 therefore, CHB is a major public health problem in Korea. Several nucleos(t)ide analogues (NAs) have been developed over the past decade, and administration of NAs has played a crucial role in the treatment of CHB. In addition to lamivudine, there are currently four oral NAs available for CHB treatment, including adefovir dipivoxil, entecavir, telbivudine, and tenofovir, which have shown greater antiviral activity compared with a placebo or lamivudine in patients with CHB.3-9
Telbivudine (β-L-2'-deoxythymidine) is a new synthetic nucleoside analogue and is a bioavailable L-nucleoside with specific anti-HBV activity in vitro.10 Since telbivudine came on the market in October 2006, a new option has been given to clinicians to treat CHB patients, especially based on the results of the GLOBE trial.11 The GLOBE trial showed that telbivudine has more potent antiviral activity and less viral resistance compared with lamivudine.11 In the preclinical toxicological tests, telbivudine had no mutagenic or carcinogenic effects.12 A minority of telbivudine-treated patients experienced creatine kinase (CK) elevation, usually transient, and myopathy occurred rarely.10 However, in our recent clinical practice, we experienced myopathy with serum CK level elevation, which seemed to be associated with telbivudine in two patients who were siblings. Myopathy is characterized by muscle pain, weakness and moderately elevated CK levels.13 We report here the two cases of telbivudine-induced myopathy in patients with CHB to consider the adverse reaction mechanisms of telbivudine.
CASE REPORT
Case 1
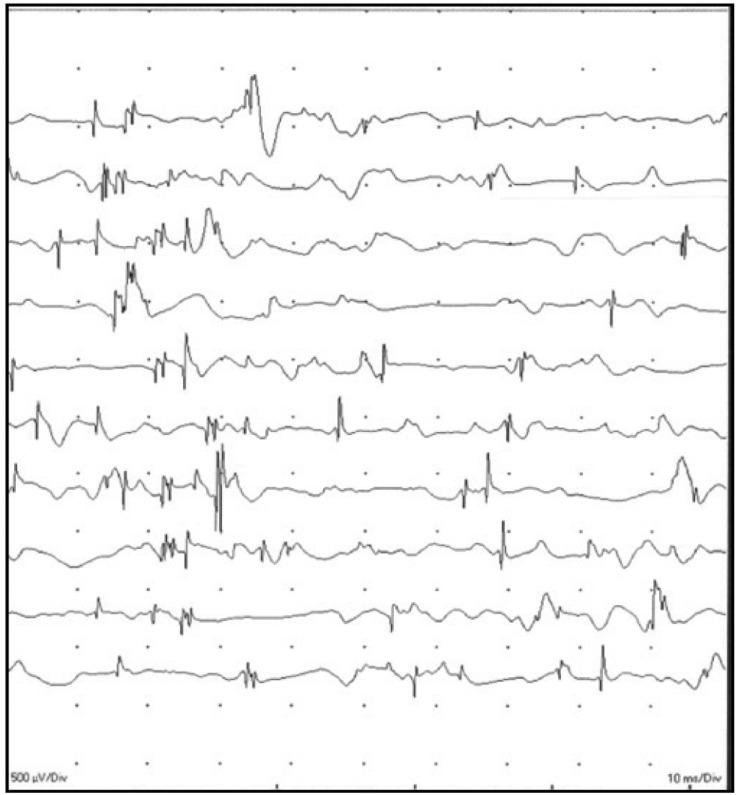
A 28-year-old man who received telbivudine therapy for chronic hepatitis B presented with progressive weakness of both lower legs and difficulty in ascending stairs over the past 4 months. And also, he was unable to lift up a dumbbell and do pushups due to weakness in both arm. He was diagnosed with CHB 3 years ago and started taking 600 mg of telbivudine once daily for 9 months to control HBV activation. Since taking telbivudine, the serum viral DNA level gradually decreased from 100,000 IU/mL to 222 IU/mL, and the serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were also normalized. However, he showed muscle weakness since taking telbivudine. Although there was no symptoms such as dysphagia, cramps, or sensory abnormality, the patient suffered from progressive and generalized weakness, especially of his legs, and had difficulty climbing stairs. He exhibited motor weakness (grade 4) of the hip flexor, making it difficult for him to stand up from the sitting position, but there was no sign of abnormal deep tendon reflex, muscle atrophy, or hypertrophy on physical examination. He had not taken any other medications that would be regarded as a cause of his symptoms while taking telbivudine. Laboratory examinations showed AST of 59 IU/L, ALT of 26 IU/L, total bilirubin of 0.9 mg/dL, blood urea nitrogen (BUN) of 15.4 mg/dL, and serum creatinine of 0.7 mg/dL. His serum CK level was elevated up to 788 IU/L (44-245 IU/L). Electromyography (EMG) was performed and showed a few positive sharp waves with polyphasic myopathic motor unit action potentials in the right vastus medialis, tibialis anterior, and biceps brachii muscles, which is consistent with the active stage of generalized myopathy (Fig. 1). We planned to perform a muscle biopsy for confirmation, but he refused to have a muscle biopsy. The serum CK level was elevated up to 728 IU/L, 4 months prior to this visit. At that time, he felt general weakness, but he did not stop taking telbivudine. Because most of telbivudine-related CK elevations were transient and increased CK levels decreased to normal range without any interruption. However, his symptoms persisted and CK elevation was also shown. Thus, he was diagnosed him with telbivudine-induced myopathy based on the clinical clues and EMG findings and decided to change the anti-viral agent to 0.5 mg entecavir once daily. He revisited our clinic at one month after telbivudine withdrawal, and his clinical symptoms subsequently improved to the extent that he was able to climb stairs easily. The serum CK level was also decreased to 326 IU/L.
Case 2
A 25-year-old man who received telbivudine therapy for chronic hepatitis B presented with progressive generalized weakness and difficulty exercising. He was diagnosed with CHB 4 years ago, and took lamivudine at first, but he changed to combination therapy of 600 mg of telbivudine once daily for 13 months and 10 mg of adefovir once to control HBV activation and lamivudine resistance. At 12 months after starting treatment, the HBV DNA level decreased to the undetectable range, and the elevated serum AST and ALT levels also decreased along with viral stabilization. However, the patient began to suffer from generalized weakness and difficult exercising since about 2 years after the treatment. He did not have dysphagia, cramps, or sensory symptoms and did not exhibit motor weakness. Laboratory analyses showed AST of 54 IU/L, ALT of 57 IU/L, total bilirubin of 0.4 mg/dL, BUN of 13.4 mg/dL, and serum creatinine of 1.15 mg/dL, and serum CK was elevated up to 2,992 IU/L. In this case, we did not conduct EMG. These two patients were siblings and they started taking telbivudine at the same time. Also, his symptoms were similar to those of his brother. His brother was diagnosed telbivudine-induced myopathy in advance. He visited the department of neurology and was diagnosed with toxic myopathy. For these reasons, we empirically diagnosed this patient with telbivudine-induced myopathy and decided to switch telbivudine to entecavir, so he received combination therapy with 0.5 mg of entecavir and 10 mg of adefovir. Two months after telbivudine withdrawal, he revisited our clinic and his laboratory test showed decreased CK level to the normal range of 157 IU/L. Also, his clinical symptoms of muscle weakness have improved.
DISCUSSION
Telbivudine, a new synthetic analogue of thymidine, is phoshporylated by host cellular kinases to telbivudine-5'-triphosphate, which has a half-life of 14 hours in vivo.10 Telbivudine-5'-triphosphate is then incorporated into HBV DNA by HBV polymerase, competing with thymidine-5'-triphosphate, the natural substrate.10 Once inserted, telbivudine-5'-triphosphate causes DNA chain termination, thereby inhibiting HBV replication.14,15
Since telbivudine was approved for the treatment of CHB, there have been a few studies11,12,14,16,17 reporting telbivudine-related adverse reactions. In the GLOBE trial, grade 3 or 4 elevation in CK levels was observed in 88 (12.9%) of 680 patients on the telbivudine arm.10,18 Myopathy, characterized by muscle pain, weakness, and elevated CK levels, was reported in two patients, which resolved after telbivudine was discontinued.12,17 Preliminary data in the GLOBE trial11 found that 9 (1.4%) patients had new onset of grade 3 or 4 CK elevations and 9 (1.4%) had myalgia or myositis between 104 and 156 weeks.11,15,19 The safety profile of telbivudine over three years was similar to that reported at two years in the GLOBE trial; most adverse effects were mild and transient in nature and no new safety signals were observed.20 The most common treatment-related adverse effects were elevated CK levels (7.5%), headache (3.6%), fatigue (2.9%), nausea (2.9%), diarrhea (2.2%), and nasopharyngitis (1.9%).20 Myalgia, myositis and muscular weakness were reported in 22 (5.3%), 2 (0.5%) and 2 (0.5%) patients, respectively.20 Xue-song Zhang et al. retrospectively reviewed five patients,18 and reported adverse reactions of telbivudine. Myalgia accompanied by general weakness, was the most common adverse reaction and the limb skeletal muscles were affected most frequently.17,19 There were four cases with peripheral nervous system involvement, represented by numbness and neuralgia, and one case with cardiac arrhythmia.12,18 While the adverse reactions of the cardiac and nervous systems persisted for a long time after discontinuation of telbivudine, myalgia resolved in a short period of time.12 Although several authors have reported regarding telbivudine-related adverse reactions from Western and Eastern countries,10-12,16 there have been no reports in Korea, especially regarding telbivudine-related adverse reactions, such as CK elevation or myopathy. To the best of our knowledge, this is the first report in Korea regarding telbivudine-related myopathy.
In these cases, the interesting point was that the two patients were siblings. The two sibling patients had started treatment for CHB around the same time and had been taking telbivudine for 9 months and 13 months, respectively. The duration of telbivudine treatment was too short compared to the other reports. This is the reason that there may be a genetic problem to telbivudine-induced myopathy. When they had myalgia symptoms, both of their CK levels were elevated to 788 IU/L and 2,992 IU/L. According to the data from our institute, 20 (7.7%) of 259 patients who have been prescribed with telbivudine showed evidence of serum CK elevation during their follow-up periods. However, in most of them, serum CK elevation did not accompany with symptoms such as muscle weakness and myopathy. Furthermore, their increased CK levels decreased to the normal range without any interruption. Although we were not able to make clear the process of telbivudine-induced myopathy because the patients did not want to have a muscle biopsy and DNA study, their elevated CK levels decreased only after telbivudine withdrawal. In addition, their symptoms were also improved after discontinuing telbivudine treatment. Therefore, we were able to conclude that myopathy in these two patients might be related to telbivudine.
In Korea, CK elevation and myopathy were reported in many cases of long-term clevudine use.21-25 Some investigators have reported that these adverse reactions are related to mitochondrial toxicity because most NAs including telbivudine as well as clevudine commonly induce mitochondrial damage by inhibiting mitochondrial DNA polymerase.18,26 Another study has suggested that the mechanism is associated with cell energy metabolism.14,18,19 Mitochondria are involved in the production of energy.18 They contain many important proteins, enzymes, and carriers that participate in energy transduction. Deficiency in any of these leads to a poor substrate supply for oxidative phosphorylation and eventually to inadequate manufacture of the energy molecule ATP, which in turn causes mitochondrial disease.18 As a competitive substrate of natural thymidine, telbivudine is phosphorylated by host mitochondrial thymidine kinases to telbivudine-5'-triphosphate and thymidine kinases are extensively exhausted.18 As a result, the normal energy transduction process is disturbed. Moreover, in the process of phosphorylation, high levels of phosphates are captured, which leads directly to exhaustion of ATP, resulting in poor energy supply.18 Nevertheless, at present, the biological mechanism of telbivudine-related CK elevations and myopathy is not yet clear. In this report, it was highly suspected that there might be a genetic problem related to this cell energy metabolism because the two patients were sibilings. Further investigation for the genetic polymorphism associated with drug clearance in these two patients would be helpful to figure out the pharmaco-dynamic relevance.
In conclusion, here we report two uncommon cases of telbivudine-induced serum CK elevation accompanied with myopathy in CHB patients. Even though there have been no cases of telbivudine-induced myopathy in Korea, physicians should take into consideration the possibility of the relationship with telbivudine as in the cases of clevudine if they were met with such an adverse reaction in their clinical practice. In addition, further closer monitoring is recommended for the evaluation of CK elevation or myopathy in patients who were treated with telbivudine.
Notes
The authors have no conflicts to disclose.
Abbreviations
HBV
hepatitis B virus
CHB
chronic hepatitis B
HCC
hepatocellular carcinoma
NAs
nucleos(t)ide analogues
CK
creatine kinase
AST
aspartate aminotransferase
ALT
alanine aminotransferase
BUN
blood urea nitrogen
EMG
electromyography