Management of portal hypertensive gastropathy and other bleeding
Article information
Abstract
A major cause of cirrhosis related morbidity and mortality is the development of variceal bleeding, a direct consequence of portal hypertension. Less common causes of gastrointestinal bleeding are peptic ulcers, malignancy, angiodysplasia, etc. Upper gastrointestinal bleeding has been classified according to the presence of a variceal or non-variceal bleeding. Although non-variceal gastrointestinal bleeding is not common in cirrhotic patients, gastroduodenal ulcers may develop as often as non-cirrhotic patients. Ulcers in cirrhotic patients may be more severe and less frequently associated with chronic intake of non-steroidal anti-inflammatory drugs, and may require more frequently endoscopic treatment. Portal hypertensive gastropathy (PHG) refers to changes in the mucosa of the stomach in patients with portal hypertension. Patients with portal hypertension may experience bleeding from the stomach, and pharmacologic or radiologic interventional procedure may be useful in preventing re-bleeding from PHG. Gastric antral vascular ectasia (GAVE) seems to be different disease entity from PHG, and endoscopic ablation can be the first-line treatment.
INTRODUCTION
Gastrointestinal (GI) bleeding is regarded as a life threatening condition in which mortality rates were reported up to 4-15%1,2,3,4 and in cases of cirrhotic patients, there are higher chances for GI bleeding compared with the non-cirrhotic patients. GI bleeding with cirrhotic patients is mostly caused by esophageal varix or gastric varix. Other causes for bleeding are gastric or duodenal ulcer similarly in cases of non-cirrhotic patients. Other minor causes for GI bleeding are portal hypertensive gastropathy (PHG) and gastric antral vascular ectasia (GAVE), etc. As the mechanisms of PHG or GAVE are still unclear and the treatment can be different according to the causes. We need more data for understanding their pathophysiology.
GI bleeding in cirrhotic patients
GI bleeding in cirrhotic patients is generally classified into variceal bleeding and non-variceal bleeding. Non-variceal bleeding is known to consist of 30-40% of total upper GI bleeding and its main causes are reported to be gastric or duodenal ulcer.5,6
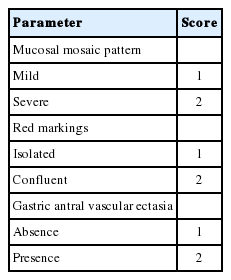
When we analyzed the data of 227 patients who visited emergency room in Dongsan hospital, Keimyung university between Jan. 2010 and Dec. 2011 due to hematemesis or melena and diagnosed as GI bleeding by endoscopic examination, 26.8% of patients were cirrhotic and the other 73.2% of patients were non-cirrhotic patients (Fig. 1).
Prevalence of gastrointestinal bleeding in patients with or without accompanying liver cirrhosis who visited emergency room at Keimyung university Dongsan hospital between Jan. 2010 and Dec. 2011. LC, liver cirrhosis.
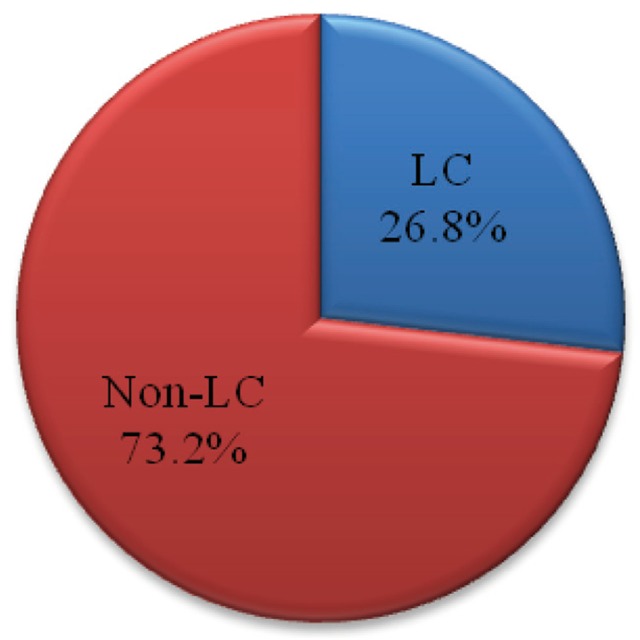
The causes of bleeding in non-cirrhotic patients were gastric ulcer (54.2%), duodenal ulcer (12.7%), Mallory-Weiss syndrome (12.7%), gastric cancer (10.8%), gastric and duodenal ulcer (5.4%), Dieulafoy's lesion (2.4%) and angiodysplasia (1.2%), etc.
In case of cirrhotic patients, the causes were esophageal varix bleeding (50%), gastric ulcer (24.2%), duodenal ulcer (7.6%), gastric varix (4.5%), etc. When ruled out the variceal bleeding, the portions of causes were gastric ulcer (64%), duodenal ulcer (20%), Mallory-Weiss syndrome (4%), gastric and duodenal ulcer (4%), etc (Fig. 2).
Analysis of gastrointestinal bleeding causes in patients with or without accompanying liver cirrhosis who visited emergency room at Keimyung university Dongsan hospital between Jan. 2010 and Dec.2011. Prevalence of bleeding causes in patients without liver cirrhosis (A) and with liver cirrhosis (B). Prevalence of non-variceal bleeding causes in patients with liver cirrhosis (C). G. ulcer, Gastric ulcer; D. ulcer, Duodenal ulcer; G. Cancer, Gastric cancer; E. varix, Esophageal varix; G. varix, Gastric varix.
The mechanisms of gastric or duodenal ulcer in the cirrhotic patients are reported to be different compared with the cases of non-cirrhotic patients. In case of non-cirrhotic patients, the main causes of ulcer bleeding are old age, intake of chronic non-steroid anti-inflammatory analgesic drugs and Helicobacter pylori infection, etc. but in case of the cirrhotic patients, the main causes were reported to be alcohol intake or portal hypertension.7,8 Besides, the bleeding can take place by PHG and GAVE.
Peptic ulcer in cirrhotic patients
The prevalence of peptic ulcer in the cirrhotic patients is reported in the range of 4.3-49%9,10 and in Korean studies, its prevalence was in the range of 24.3% to 38.3% which shows higher compared with the reports from western countries.11,12
In liver cirrhosis, the incidence of peptic ulcer increases, longer treatment duration is needed when treat with H2 receptor antagonists, and, the higher recurrence rate has been reported within a year compared with the non-cirrhotic patients (17-20% vs. 21-54.5%).9
As congestion and impaired angiogenesis are caused by change in the microcirculation of gastric mucosa due to portal hypertension, the defense mechanisms for gastric acid or bile reflux, etc. are impaired in the cirrhotic patients.13,14
Helicobacter pylori infection is reported to play an important role as the risk factor of peptic ulcer development in cirrhotic patients.10 However, its recurrence patterns in cirrhotic patients after eradication of Helicobacter pylori showed somewhat different from the non-cirrhotic patients. Recurrence rates of duodenal ulcer after one year of eradication in cirrhotic patients was 58%15 and also another study reported the 41% of ulcer recurrence rate within 2 years after eradication in cirrhotic patients. So, these data show that the risk factors of the recurrence of peptic ulcer in cirrhotic patients would be correlated with degree of impaired liver function or the presence of variceal bleeding rather than the success of Helicobacter pylori eradication.16
Portal hypertensive gastropathy in cirrhotic patients
PHG is the change in the gastric mucosa of the patient with portal hypertension, and mucosal changes of PHG can be defined as the presence of mucosal friability and dilated blood vessels in the mucosal surface (Fig. 3).
The frequency of PHG has been variously reported in the range of 9-80% according to each studies since the coherent diagnostic criteria was not available.17,18,19,20 The severity of PHG may be positively correlated with the disease duration, the presence of esophageal or gastric varix, size of varix and the history of sclerotherapy. But actually, incidence of GI bleeding in PHG is relatively rare and the cases to cause severe GI bleeding are extremely rare.21
Recently, the diagnosis for PHG or classification of its severity is carried out by the 2-step classification suggested by Baveno consensus workshop (Table 1).22 While keeping track of its progress for 18 months on average, about 29% of cases showed no significant change, 23% or so showed significant progression, 23% or so showed improvement and other one fourth of cases showed wax and wane depends on patients situation.21
Regurgitant blood flow to the stomach due to portal hypertension is considered as primary cause of PHG.17,18,19,20 However, the relationship between increased level of portal blood pressure and the severity of PHG is still unclear.17,18,23 The imbalance between offense factors and defense factors of gastric mucosa can be the other cause of development of clinically significant GI bleeding in PHG.18
It is reported that about 10% of PHG cause anemia due to the chronic blood loss, 2.5% of patients experienced acute bleeding. Medications for reducing portal blood pressure may be needed in some selective patients because mortality rate related to acute GI bleeding reaches of 12.5%.21 Nonselective beta blockers are reported to improve the patients' quality of life by reducing the severity of PHG and to prevent rebleeding.24 Medications such as octreotide and somatostatin used for the treatment of portal hypertension are reported to be very effective for control of acute GI bleeding.25 In additions, transjugular intrahepatic portosystemic shunt formation which reduce portal blood pressure is also reported to improve the severity of PHG and to decrease the frequency of rebleeding.26
Because relationship between Helicobacter pylori infection and PHG is not clearly identified, the proper evidence about need of eradication for the management of PHG is insufficient.27 In addition, the evidences of proton pump inhibitors to reduce the frequency of ulcer formation and related bleeding caused by PHG are to be proved.28
Gastric antral vascular ectasia in cirrhotic patients
GAVE is a cause of chronic GI bleeding and iron deficiency anemia while related to the expansion of small blood vessels in gastric antrum. Dilated blood vessels are observed in the form of red straight line at the antrum under endoscopic examination. And pathologically, the specific findings such as dilated capillaries, fibromuscular dysplasia, fibrin thrombus, and fibrohyalinosis are observed.29
Compared with body part in case of PHG, affected site is gastric antrum in GAVE. Treatment to reduce the portal blood pressure can improve the PHG. But decrease of portal blood flow cannot improve the GAVE. Therefore, PHG and GAVE may be classified into the different disease category.30,31,32
About 30% of patients with GAVE also are cirrhotic29 and around 2.5% of decompensated cirrhotic patients who need liver transplantation are reported to have the GAVE.30,31,32
The change of gastric antral motility and vasoactive materials may be expected to be involved in the mechanism of GAVE, but that is not clearly proved. Local treatment such as argon plasma coagulation therapy through endoscopic procedure can be applied for the management of GAVE.33 For the patients who are not effectively controlled by local therapy, the antrectomy or the hormone therapy such as estrogen or progesteron can be considered as additional treatment.30,31,34
CONCLUSION
Cirrhotic patients experience more frequent GI bleeding episodes compared with non-cirrhotic patients. Major causes of GI bleeding in cirrhotic patients are esophageal varix and gastric varix bleeding. Other causes are gastric and duodenal ulcer as similar with non-cirrhotic patients. Besides, PHG and GAVE are considered as other causes of GI bleeding in cirrhotic patients. The mechanisms of these two diseases are not clearly identified and their management can be different accordingly. So, more precise understanding of these conditions is still needed.
Notes
The author has no conflicts to disclose.
Abbreviations
GAVE
Gastric antral vascular ectasia
GI
Gastrointestinal
PHG
Portal hypertensive gastropathy