Alcoholic fatty liver disease elevates estimated coronary heart disease risk to levels comparable with those of nonalcoholic fatty liver disease in the Korean population: a cross-sectional study
Article information
Abstract
Background/Aims
A close relationship has been established between nonalcoholic fatty liver disease (NAFLD) and an elevated risk of coronary heart disease (CHD), but little is known about the association between alcoholic fatty liver disease (AFLD) and CHD risk. The aim of this study was to determine whether AFLD is associated with elevated CHD risk.
Methods
We retrospectively enrolled 10,710 subjects out of 11,469 individuals who visited the Konkuk University Health Care Center for a routine health checkup in 2010. AFLD was diagnosed made when the usual amount of alcohol consumption exceeded 210 g/week in males and 140 g/week in females for the previous 2 years and when hepatic steatosis was detected by liver ultrasonography. The 10-year risk for CHD was estimated using the Framingham Risk Score.
Results
Hepatic steatosis was diagnosed in 4,142 of the 10,710 individuals (38.7%); the remainder (i.e., n=6,568) became the control group. The 4,142 individuals with hepatic steatosis were divided into two groups: NAFLD (n=2,953) and AFLD (n=1,189). The risk of CHD was higher in AFLD (6.72±0.12) than in the control group (5.50±0.04, P<0.001), and comparable to that in NAFLD (7.32±0.07, P=0.02).
Conclusions
Individuals with AFLD have an elevated 10-year risk of CHD that is comparable to those with NAFLD. Therefore, AFLD should be considered a significant risk for future CHD, and preventive measures should be considered earlier.
INTRODUCTION
Fatty liver represents a common clinical entity that can be divided into alcoholic fatty liver disease (AFLD), and nonalcoholic fatty liver disease (NAFLD). Both are within the most common causes of chronic liver disease worldwide, and are linked to the rising incidence of obesity, diabetes and coronary heart disease.1 Only 3% of individuals with NAFLD progress to fibrosis or cirrhosis,2 whereas 5-15% of subjects with AFLD develop cirrhosis, despite abstinence.1
The prevalence of alcohol consumption is 90% in men with age between 40-49, 88% of men of all age and 70% of women over 19 years consume alcohol representing 79% of total Korean adult population.3 In addition, about 14% of Koreans meet DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition) criteria4 for alcohol use disorder during their lifetime.5 Furthermore, the prevalence of high risk drinking is 15% in Korea,6 higher than that in Canada (9.5%), France (5.3%), and the US (8.2%) as well as the global rate of 11.5%.7 Alcohol consumption creates various disturbances in metabolic functions and tissue damage in almost every organ of the body.8 Although a light to moderate amount of alcohol is reported to decrease risk of coronary artery disease and cardiovascular mortality,9 significant alcohol consumption is associated with an increased risk of hypertension in a dose-dependent manner.10 Furthermore, long-term alcohol consumption may increase the prevalence of central obesity, metabolic syndrome (MS), and cardiovascular disease (CVD).11,12 Excess alcohol increases CVD risk because the beneficial increase in high-density lipoprotein cholesterol (HDL-C) is offset by increased blood pressure levels.13 However, despite its high prevalence and the substantial side effects involved, there has been limited research into AFLD not only in Korea but also on the international level.14 Alcohol is a causal factor in 60 types of diseases and injuries and a component cause in 200 others.2 However, we have little understanding of the negative consequences of AFLD, except for its cirrhotic complications.
Our objective was to determine whether those with AFLD have an elevated 10-year risk of developing CHD, to investigate the relationship between AFLD and CHD risk, and to compare this risk with that for NAFLD population and to assess non-inferiority of AFLD compared with NAFLD for cardiovascular risk.
MATERIALS AND METHODS
Study design
This is a cross-sectional study of the association between AFLD and CHD risk. We performed the retrospective analysis of the data collected in 2010, at a single center of a region, Konkuk University Health Care Center in Seoul. The recruitment period of the individuals who visited Konkuk University Health Care Center for general health check-up was from January 2010 to December 2010. The individuals visited the hospital and took the medical examination on just one day. They completed the self-administered questionnaire that had been sent by mail several days before and submitted it on the visiting day. The results of the examinations were noticed to the individuals by mailing or phone calls. The health care program included several different types of examinations upon the individuals' requests. Study data included laboratory, physical examination, liver US finding and information of the questionnaire.
Study samples
Patients were considered eligible if the three following inclusion criteria were fulfilled: (a) age 18 years or older, (b) subjects who visited Konkuk University Hospital health care center between January 2010 and December 2010 and who had undergone liver US, (c) individuals who had fulfilled the self-administered questionnaire. The study participants reached 11,469 individuals. Out of them, we excluded 167 participants with at least one potential cause of chronic liver disease: 98 subjects with hepatitis B virus, 25 subjects with hepatitis C virus, 9 subjects with some other history of hepatitis. In addition we excluded 35 participants with history of CHD. Altogether, 10,710 individuals were included in the study. Study protocol was approved by the Institutional Review Board of Konkuk University Hospital at Seoul and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Clinical and laboratory evaluations
We reviewed all the medical records performed at health check-up, including anthropometric, laboratory and imaging study. The anthropometric data included systolic and diastolic blood pressure, height, weight, waist circumference. There was also information about participants' past history, family history, drug history and alcohol, smoking history, all collected by self-administered questionnaire. We abstracted all this information including the questionnaire from the medical record. Regarding to alcohol history, the self-administered questionnaire included the following two questions: (a) How often do you have a drink containing alcohol, during an average week (b) How many units of alcohol have you drunk during a typical occasion over the past 12 months The weekly frequency was evaluated using a closed question comprising six subcategories where patients were to choose one of the following answers: (a) once daily (b) 5-6 times per week (c) 3-4 times per week (d) 1-2 times per week (e) 2-3 times per month (f) less than once a month. The standard number of drinks consumed on an average occasion was measured by an open question. We defined significant alcohol consumption based on the threshold of ≥210 g/week (men) and ≥140 g/week (women) and discriminated between NAFLD and AFLD accordingly (15). A standard drink was defined as having 10 g of ethanol, which comprises 150 mL of wine, 200 mL of beer, 50 mL of spirit, or 50 mL of Soju, the most popular alcoholic beverage among Koreans.14 The total amount of alcohol consumed per week on average was obtained by multiplying the weekly frequency ((a) 7 for once daily (b) 5.5 for 5-6 times per week (c) 3.5 for 3-4 times per week (d) 1.5 for 1-2 times per week (e) 0.75 for 2-3 times per month (f) 0.25 for less than once a month) by the consumption amount on a single occasion, at 10 g per drink. The amount of alcohol consumption is expressed in gram/week.
Laboratory data included aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (rGT), serum total cholesterol, triglyceride, HDL-C, fasting plasma glucose, C-reactive protein and ferritin.
Diagnosis of NAFLD and AFLD
Hepatic steatosis was diagnosed if he/she had the following components at liver US: Ultrasound beam attenuation and poor visualization of intra-hepatic vessel borders and diaphragm, evidence of diffuse hyper-echogenicity of liver relative to kidneys. Liver US was performed in all patients by two experienced radiologists. The intra-observer variability for the ultrasound diagnosis of hepatic steatosis was within 3%.15 We diagnosed NAFLD by relying on the presence of hepatic steatosis as confirmed by liver US when the following criteria were absent: a) excess alcohol consumption defined as >210 g/week in men and >140 g/week in women based on the KASL definition, b) causes for secondary hepatic fat accumulation such as steatogenic medications or hereditary disorders c) positivity for hepatitis B surface antigen or antibody to hepatitis C virus. AFLD was diagnosed based on a combination of features including ongoing or recent excessive alcohol consumption with hepatic steatosis confirmed by liver US. Two radiologists performed US scanning using the Philips model (iU22x MATRIX Ultrasound System, Philips). Definition of metabolic syndrome was based on the World Health Organization-West Pacific Region Guideline16 and Insulin resistance was estimated by homeostasis model assessment of insulin resistance.17
CHD risk assessment
Among various Framingham-based tools, we used the conventional Framingham risk score (FRS) from Wilson et al 199818 to estimate total CHD risk at 10-year. For hard CHD risk estimation, we used the revised FRS form, where ATP III risk factor scoring is incorporated into the 10-year risk assessment tool.19 The FRS was calculated based on six coronary risk factors: gender, age, total cholesterol, HDL-C, systolic BP and smoking habit. Among these factors, age, BP, and cholesterol levels were categorized according to their values and smoking status was classified as either "current smoker" or "non-smoker". Finally, the corresponding point was assigned to each individual and the total score was used as the individual's CHD risk level.19
Statistical analysis
Quantitative data was expressed as mean±standard deviation (SD), and categorical data as percentages. We used chi-square tests or the Mann-Whitney test and analysis of variance or Kruskal-Wallis test for continuous variables. One-way analysis of covariance (ANCOVA) was used to compare the means of FRS and 10-year risk for total and hard CHD between the control and fatty liver group, and between the NAFLD and AFLD group after adjustment for the main differences between the groups (age, sex, metabolic syndrome, smoking and family history of CHD). All statistical tests were two-sided, and a P value of less than 0.05 was considered to be significant. Statistical analysis of the data was performed using SPSS version 17.0 (SPSS Inc., Chicago, Ill, USA).
RESULTS
Baseline characteristics
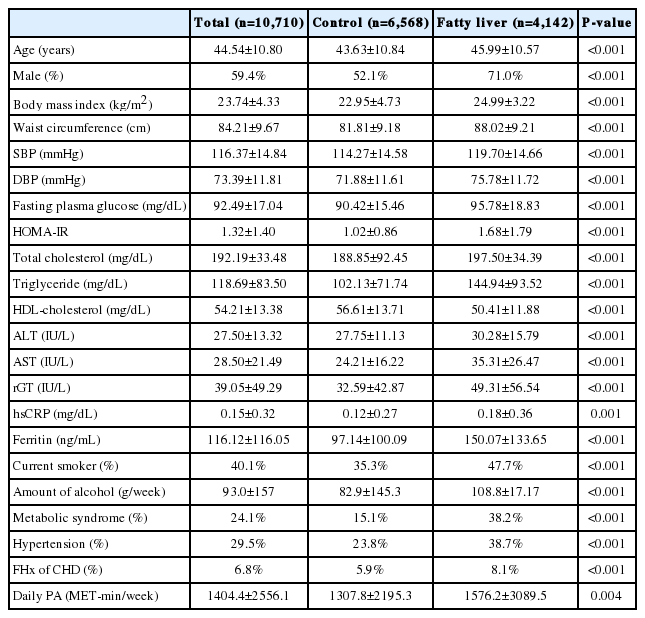
Out of 11,469 individuals, 10,710 subjects met the inclusion criteria. The characteristics of the study population are shown in Table 1. Of 10,710 individuals, 59.4% were men with an average age of 44.5 and a BMI of 23.8 kg/m2, for whom the prevalence of metabolic syndrome was 24.1%. Relying on liver US, we discriminated 4,142 fatty liver subjects (38.7%), with the remaining 6,568 (61.3%) constituting the control group. The majority of overall group comparisons showed significant differences, with higher mean values in the population with fatty liver as compared to the control group, except for waist-to-hip ratio (P=0.08) and HDL-C which was higher in the control group (P<0.001).
NAFLD vs. AFLD
Of the 4,142 subjects with fatty liver, 71.3% (2,953) had NAFLD and 28.7% (1,189) had AFLD. Table 2 shows the comparisons of the baseline characteristics between NAFLD and AFLD. Subjects with AFLD were more likely to be younger (46.2 vs. 44.3 years, P<0.001), men (62.4 vs. 92.3%, P<0.001), and were also more likely to be smokers (36.8 vs.74.6%, P<0.001), with all these variables showing significant differences compared to the NAFLD group. Subjects with NAFLD and AFLD showed comparable results for HOMA-IR, plasma level of HDL-C, low-density lipoprotein cholesterol (LDL-C), serum high-sensitive C-reactive protein (hsCRP) and daily physical activity. There were statistical differences in mean blood pressure, fasting plasma glucose, serum AST, ALT, and ferritin between the two groups.
Comparison of CHD risk between groups
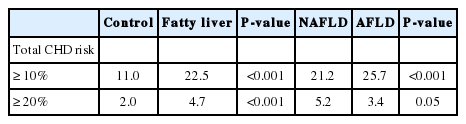
Table 3 shows comparisons of mean 10-year estimated total risk between the control and fatty liver group, and the NAFLD and AFLD group. The mean 10-year risk for total CHD was higher in the fatty liver group than the matched control group. Individuals with AFLD had a higher 10-year estimated CHD risk compared to the control group (6.72±0.12 vs 5.50±0.04 P<0.001) and a comparable risk to those with NAFLD (6.72±0.12 vs 7.32±0.07, P=0.02). The prevalence of total CHD risk over 10% (more than moderate risk) and over 20% (high risk) were significantly higher in the fatty liver group as compared to the control. However, between NAFLD and AFLD, we obtained inconsistent results showing a higher prevalence of total CHD events greater than 10% in the AFLD group, whereas total CHD over 20% showed no unidirectional tendency (Table 4).
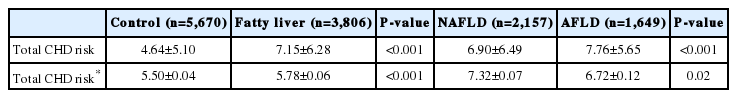
Comparisons of mean estimated 10-year total CHD risk in fatty liver vs. control group and NAFLD vs AFLD group
DISCUSSION
The aim of the current study was to determine whether subjects with AFLD have an elevated CHD risk using FRS and to compare it with that for NAFLD. We found a strong association between AFLD and CHD risk comparable to NAFLD. In addition, AFLD was a strong predictor elevated CHD risk (CHD risk >10%) independent of traditional risk factors.
To our knowledge, this is the first attempt to measure CHD risk in AFLD and to compare it with that of NAFLD. Although there are many reports for NAFLD population on this topic,20,21 few exists on AFLD population. Only study comparing the frequency of coronary artery disease in alcohol-related versus non-alcoholic end-stage liver disease (ESLD) is available in the literature.22 In this study, 420 patients with ESLD either alcohol-related or non-alcohol related, underwent coronary angiography for candidate screening of liver transplantation. And a significantly higher proportion of patients in the alcohol-related group had coronary artery disease compared to non-alcohol group (P<0.01). This difference was observed despite these groups otherwise being comparable in terms of age and CAD risk factors. Their report has some disparities with our results. However the overall study population in this analysis had advanced liver diseases with substantially different baseline metabolic risk profiles when compared to our study population, making it inappropriate to directly compare the results. Another research by JH Kim and colleagues that examines 265 fatty liver subjects has recently been published providing comparison of carotid intima-media thickness (cIMT) between individuals with NAFLD and AFLD.23 The relationship between increasing cIMT and incident CVD events has been clearly established24 and several prospective, population-based studies demonstrated that the presence of carotid plaque is associated with a significantly increased risk for myocardial infarction, stroke and CHD death, independent of traditional risk factors.25 In this study, cIMT was elevated in subjects with fatty liver in both NAFLD and AFLD patients, in agreement with our results.
In the current study, we demonstrated the prevalence of both NAFLD and AFLD in apparently healthy individuals. The prevalence of NAFLD based on liver US was 27.6% in our study, consistent with the previous reports between 17 and 46%.26,27 Although reference data on the prevalence of AFLD is limited, there is a study using the population-based FIN-D2D survey, in which a 7.0% of prevalence is reported.28 We found a higher prevalence of AFLD (11.1%), with this discrepancy possibly due to the high alcohol consumption rate in the Korean population. As mentioned earlier, compared to the NAFLD group, the AFLD group is comprised individuals with younger age, male sex, current smoking status, central obesity and metabolic syndrome. These findings agree well with several previous reports that excess alcohol consumption is more prevalent in males29 with combined smoking habits. Indeed 90% of all alcoholics smoke and alcoholism is ten times more prevalent among smokers than non-smokers.30 However the fact that the prevalence of essential hypertension, and metabolic syndrome is higher in individuals with AFLD compared to NAFLD suggests the new possibility that in some AFLD subjects, the CVD risk may be higher than our expectation and further investigation should be conducted.
Another important strength of the current article is that it is the first study comparing NAFLD and AFLD populations in terms of CHD risk and metabolic profiles. There has been accumulating evidence for a strong association between NAFLD and elevated CHD risk over the last years.31 Although the pathogenesis behind this link has not been fully elucidated, the contributions of obesity and metabolic syndrome to increased CHD events are well known.21,32 By contrast, the association of AFLD with CHD risk had not been investigated. In this study, we have demonstrated a significant increase in 10-year CHD risk not only in subjects with NAFLD, but also those with AFLD, a close association that was independent of age, sex, HOMA-IR, or the individual components of metabolic syndrome, implying that AFLD is a strong independent predictor for developing CHD events within 10 years.
The current study benefitted from the fact that its results were based on a large population. It is also significant that we compared subjects with NAFLD and AFLD in terms of future CHD risk by estimating 10 year total CHD risk. This study is conducted only in Koreans. Considering its high alcohol consumption rate, racial difference and genetic variances, the conclusion may not be applicable widely up to other countries. Secondly, we diagnosed hepatic steatosis based on liver US instead of histological tissue confirmation. Although this is better than using a definition on elevated transaminase as a proxy for the diagnosis, it is still a surrogate for the diagnosis and is not completely sensitive in cases with milder liver steatosis. Thus mild steatosis (mild NAFLD or AFLD) might have been included in the control group. To correct for this, a prospectively designed studies are warranted. Thirdly as this is a large retrospective cohort study, we could only estimate future CHD risk using FRS based on laboratory and clinical data. Additionally, the alcohol consumption was measured by self-reported questionnaire participants so this study may show selection bias. Regardless of these limitations, strengths of the present study include the large sample size of healthy male participants with comprehensive measurements of liver, including the direct measurement of estimated cardiovascular risk factors.
CONCLUSIONS
In summary, subjects with fatty liver had significantly higher estimated 10-year total CHD risk compared to the control group, not only for NAFLD but also for AFLD. Therefore, AFLD should be considered as a meaningful risk for future CHD events and preventive measures should be considered earlier.
Notes
The authors have no conflicts to disclose.
Abbreviations
AFLD
Alcoholic Fatty Liver Disease
CHD
Coronary Heart Disease
FRS
Framingham Risk Score
NAFLD
Nonalcoholic Fatty liver disease