| Clin Mol Hepatol > Volume 20(2); 2014 > Article |
ABSTRACT
Background/Aims
Adefovir dipivoxil (ADV) is a nucleotide analogue that is effective against lamivudine-resistant hepatitis B virus (HBV). The aim of this study was to determine the long-term clinical outcomes after ADV rescue therapy in decompensated patients infected with lamivudine-resistant HBV.
Methods
In total, 128 patients with a decompensated state and lamivudine-resistant HBV were treated with ADV at a dosage of 10 mg/day for a median of 33 months in this multicenter cohort study.
Results
Following ADV treatment, 86 (72.3%) of 119 patients experienced a decrease in Child-Pugh score of at least 2 points, and the overall end-stage liver disease score decreased from 16±5 to 14±10 (mean ± SD, P<0.001) during the follow-up period. With ADV treatment, 67 patients (56.3%) had undetectable serum HBV DNA (detection limit, 0.5 pg/mL). Virologic breakthrough occurred in 38 patients (36.1%) and 9 patients had a suboptimal ADV response. The overall survival rate was 89.9% (107/119), and a suboptimal response to ADV treatment was associated with both no improvement in Child-Pugh score (≥2 points; P=0.001) and high mortality following ADV rescue therapy (P=0.012).
About 350 million people worldwide have chronic hepatitis B virus (HBV) infection,1 and these individuals are at increased risk of developing hepatic complications, such as cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).2 The ultimate goals of HBV treatment are to induce remission and to prevent such complications by sustained suppression of HBV replication.3 Interferons, including pegylated interferons, and nucleotide analogues are regarded as standard treatment for patients with chronic hepatitis B, and nucleotide analogues have been shown effective and safe in chronically infected patients with decompensated cirrhosis.3,4,5,6,7,8,9,10,11 Treatment with the nucleotide analogue lamivudine has been shown to improve liver function (Child-Pugh class) and to prolong survival in patients with decompensation.4,5,7,8,9,11 In addition, earlier administration of antiviral drugs (that is, to patients with Child-Pugh B) leads to better prognosis than later administration to patients with poor liver function (that is, Child-Pugh C).11
Over time, however, most patients experience resistance to long-term lamivudine therapy.12,13 Adefovir dipivoxil (ADV) is another orally bioavailable nucleotide analogue, effective against wild-type and lamivudine-resistant HBV in vitro and in the clinic.14,15,16,17,18,19 Now, the outcome of ADV rescue therapy in chronic hepatitis patients with lamivudine resistance can be seen in practice guideline for hepatitis B through many clinical studies.3,20 In addition, short-term treatment with 10 mg/day ADV of patients with decompensated cirrhosis and lamivudine resistance have been associated with favorable virologic and biochemical responses.17,18,19 Less is known, however, about the long-term outcomes associated with ADV rescue therapy in decompensated patients with lamivudine-resistant HBV.19 We have therefore assessed long-term clinical outcomes after ADV rescue therapy in decompensated patients infected with lamivudine-resistant HBV.
This retrospective, multi-center cohort study assessed 128 decompensated liver cirrhosis patients with lamivudine-resistant hepatitis B virus who were treated with ADV 10 mg/day through the Adefovir depivoxil Compassionate Access Program (ACAP), starting in May 2003. Between May 2003 and March 2004, total 202 patients were screened at 11 hospitals in Korea. Of these, 74 patients were excluded from the analysis owing to compensated hepatic function (n=18), baseline HCC (n=17), post-liver transplantation state (n=16), refusal of informed consent (n=6), and death before ADV treatment (n=17) (Fig. 1). At the time of this study, no antiviral therapy other than ADV was available in Korea for treatment of lamivudine-resistant HBV. The presence of lamivudine-resistant HBV was confirmed by restriction fragment length polymorphism analysis.21 Patients remained in the study until ADV became available commercially. Decompensation was defined as a Child-Pugh (CP) score ≥ 7, together with a history of cirrhotic complications, including ascites, variceal hemorrhage, hepatic encephalopathy or spontaneous bacterial peritonitis.17,22
Concomitant lamivudine treatment was allowed and was discontinued at the discretion of the investigator. Patients with reduced renal function were managed by adjusting the dosing interval of ADV, based on creatinine clearance measurements.
Factors assessed at baseline included complete blood chemistry; serum electrolyte concentrations; serum ALT, bilirubin, and albumin concentrations; international normalized ratio (INR) for prothrombin time; presence of cirrhotic complications, including hepatic encephalopathy, ascites, variceal hemorrhage or spontaneous bacterial peritonitis; and hepatitis B serology, including HBsAg, anti-HBs, HBeAg, anti-HBe, and serum HBV DNA concentrations (Digene Hybrid Capture II assay, detection limit, 0.5 pg/mL). HBV serology, including HBV DNA concentration, and serum electrolytes, including serum creatinine and phosphorus concentrations, were measured regularly. Resistance surveillance for ADV was not routinely performed. All laboratory data were based on local references and assessed centrally.
All patients in the study signed the informed consent form before enrollment. The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by each Institutional Review Board.
The end of follow-up was patient death, time of liver transplantation or last visit. Following outcomes were analyzed: changes in CP scores and model for end-stage liver disease (MELD); the proportion of patients with normalized ALT, serum bilirubin and albumin concentrations, and prothrombin time (INR); the proportion of patients with undetectable serum HBV DNA during ADV treatment; the frequency of liver transplantation and overall patient survival. Antiviral responses, including virologic breakthrough, suboptimal response, and virologic relapse, were also evaluated. Virologic breakthrough was defined as a detectable serum HBV DNA during treatment after achieving serum HBV DNA undetectability; suboptimal response was defined as sustained detectable HBV DNA after at least 6 months of continued treatment; and virologic relapse was defined as an reappearance of detectable serum HBV DNA after discontinuation of treatment.3,8,23
Descriptive statistics were reported as mean ± standard deviation or median (range) unless indicated otherwise. Cumulative rate of patient's events was analyzed using the Kaplan-Meier method. Changes in biochemical variables from baseline were tested using a 1-sample t-test and differences between subgroups were compared using the chi-square test, or one way ANOVA test as appropriate. Factors associated with clinical outcomes were investigated using Cox proportional regression analysis. Any variables identified as significant (P<0.1) in univariate analysis were included in multivariate analysis. Two-sided tests with P<0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 14.0 software (SPSS, Chicago, IL, USA).
The baseline characteristics of the 128 patients at the start of ADV treatment are shown in Table 1. Their mean age was 49.7±8.2 years and 97 patients (76%) were males. One hundred patients (78%) were positive for HBeAg at baseline and their mean HBV DNA concentration was 597±1039 pg/ml. None of the patients had HIV or hepatitis C virus co-infection. The mean duration of lamivudine treatment prior to ADV treatment was 36.2±17.6 months. Out of total patients (n=128), lamivudine was stopped after initiation of ADV therapy in 53 patients (41.4%). In the other 75 patients (58.6%), lamivudine was switched to ADV therapy with a mean 7.6±5.2 months of overlapping period. Of these 75 patients, 7 patients maintained lamivudine with concomitant ADV until the end of follow-up. Baseline hepatic function was CP class B in 59% and CP class C in 41%, and their mean MELD score was 16.6±5.6 (Table 1).
Median follow-up period in the 128 patients was 33 months (range 1-41 months). The analysis of clinical efficacy was analyzed after excluding patients who received liver transplantation (n=9) before ADV therapy and who discontinued ADV early (n=10). Following treatment, CP score decreased by ≥2 points in 86 patients (78.9%), was unchanged in 16 patients (14.7%), and increased by ≥2 points in 7 patients (6.4%). Of the 67 patients with CP score B at baseline, 57 (85%) improved to CP score A, 6 (9%) remained at CP score B, and 4 (6%) deteriorated to CP score C. Of the 42 patients with CP score C at baseline, 29 (69%) improved to CP scores A (23, 55%) and B (6, 14%), while 13 (31%) remained at CP score C (P=0.001) (Fig. 2). Overall mean MELD score decreased from 16±5 to 14±10 (P<0.001). ALT was normalized in 74%, albumin in 67%, bilirubin in 70% and prothrombin time (INR) in 67.5%.
At baseline, all patients had HBV DNA concentrations >0.5 pg/mL. Throughout ADV treatment period, undetectable HBV DNA (<0.5 pg/mL) was achieved and maintained in 67 (56.3%). During the ADV treatment, 38 of 119 (31.9%) showed virologic breakthrough during a median follow-up of 33 months; the cumulative incidence of virologic breakthrough was 6.5%, 15%, and 32.5% at 12, 24, and 36 months, respectively. Nine of 119 (7.6%) patients showed a suboptimal response to ADV treatment, as shown by sustained detectable HBV DNA (≥0.5 pg/mL) during a median 33 months (range 1-41 months). Among various clinical factors, virologic response to ADV (HBV DNA undetectability and virologic breakthrough vs. suboptimal response, 90.2%, 82.9% vs. 25%; P=0.001 Odds ratio 3.984 (95% confidence interval (C.I.) 2.262-7.017) was shown to be independently associated with improvement of CP score (≥2 points) by multivariate analysis (Table 2).
The virologic response to ADV treatment (HBV DNA undetectability, virologic breakthrough, suboptimal response) was not associated with concomitant lamivudine treatment (switch without overlapping vs. switch with overlapping vs. add-on, P=0.852) or with the duration of previous lamivudine treatment (P=0.664) and other baseline characteristics were not different among patients with different virologic response except ALT level (Table 3). Of 7 patients with add-on ADV-lamivudine treatment, two patient diagnosed with HCC later had detectable HBV DNA at 6, 14 months of treatment (1 virologic breakthrough, 1 suboptimal response), respectively.
During the study period, nine patients underwent liver transplantation. Among the remaining 119 patients, 12 (10.1%) patients died. The cumulative 6-month, 1-year and 2-year survival rates were 95.8%, 94.1% and 89.6% (Fig. 3). Of the 12 patients who died, all patients except two patients were in CP class C at their enrollment. Five patients who died within 1-2 months of treatment without improvement in liver function had baseline MELD score ≥25 (Table 4). Two patients with CP class B (MELD score, 15 and 10, respectively) died at 6 months and 23 months after treatment, both due to HCC and liver failure. The remaining five patients died at a median of 17 months (range 6-23 months) after the start of treatment. These five patients showed unfavorable virologic responses; two patients had suboptimal response, two had virologic breakthrough and one had virologic relapse.
Survival rate following ADV rescue therapy was different according to the virologic response to ADV treatment; patient with suboptimal response to ADV treatment showed significantly lower overall survival rate than with sustained virologic suppression or with virologic breakthrough (66.7% vs. 98%, 94.7%, respectively, P=0.012) (Fig. 4).
ADV was well tolerated in all 128 patients throughout the treatment period, with no serious side effects. During follow-up, the mean change in serum creatinine from baseline was 0.04±1.51 mg/dl (P=0.242). Ten of 98 patients (10.2%) showed ≥0.5 mg/dl increases in serum creatinine, with 6 patients discontinuing treatment due to death from liver failure. All six of these patients had baseline CP class C scores and their median MELD score was 22 (range 16-36). In the remaining four patients, the serum creatinine level did not exceed 1.5 mg/dl and all continued treatment until the end of follow-up. Serum phosphorus level did not decrease to less than 2 mg/dl during treatment.
This multicenter long-term cohort study assessed the effects of ADV treatment in 128 decompensated patients with lamivudine-resistant HBV in an area in which HBV infections are highly endemic. Our findings strongly support previous results, showing that ADV rescue therapy was associated with virological, serological, and CP improvement, as well as with survival benefit in decompensated patients with lamivudine-resistant HBV.17,18,19 In the previous studies, however, the follow-up period was relatively short (<12 months), and they involved a small number of recruited patients. In contrast, our patients had more severe liver dysfunction, all of CP class B (59%) and C (41%), and we used a longer follow-up period (median, 33 months; range 1-41 months), even when compared with a recent study of 226 patients, 25% with CP class A, who were followed for a mean of 37 weeks while awaiting liver transplantation in the United States.19 To our knowledge, this study is the first to report long-term (approximately 3 years) results of ADV rescue treatment for decompensated patients with lamivudine-resistant HBV infection.
Antiviral treatment has been found to improve CP score in decompensated patients with HBV-infection.4,5,9,11 We found that ADV treatment decreased CP score by at least 2 points in 78.9% of patients, had no effect in 14.7%, and increased CP score by at least 2 points in 6.4%. We found that 85% of patients with CP class B at baseline improved to CP class A, whereas 69% patients with CP class C improved to CP classes A and B, further suggesting that CP class C liver function at baseline is associated with poor clinical response to antiviral therapy in patients with HBV-related decompensation. Therefore, similar to baseline liver function at the start of lamivudine therapy,11 baseline function at the initiation of ADV may predict response to treatment.
In this study, the virologic response to ADV treatment was associated not only CP improvement but also survival rate following ADV treatment (HBV DNA undetectability, virologic breakthrough vs. suboptimal response). Previous study showed that antiviral therapy has been shown to slow disease progression only in patients with maintained viral suppression.24 In contrast, failure of antiviral treatment had no impact on survival of HBV patients awaiting orthotropic liver transplantation.23 The baseline liver function of patients enrolled in that study, however, was better: 68% of patients had low MELD scores. Interestingly, in the result, patients with virologic breakthrough following ADV treatment did not show poor clinical outcome as with suboptimal response. It may be because the observed duration following virologic breakthrough was not long enough to see the clinical consequences.
Out of 12 ADV-treated patients who died during the study period, 5 patients died within 1-2 months of treatment had baseline MELD score≥25. This finding is consistent with those of previous studies showing that the pretreatment severity of decompensated liver cirrhosis is the most important predictor of early mortality.8,25,26,27 Clinical improvement would not be expected in these patients because they already had severe decompensation before ADV treatment. However, other antiviral agent such as tenofovir, known as more potent antiviral agent in lamivudine resistant HBV than ADV,28,29 might suppress viral replication more rapidly and completely and would result in different outcome in these decompensated patients.
A major concern during antiviral treatment in patients with decompensation is the appearance of drug-resistant HBV. In lamivudine-resistant patients, the cumulative incidence of ADV mutations 24 months after switching from lamivudine to ADV was more than 25%, whereas the 2-year incidence in naïve patients receiving ADV therapy was 3%.16,30,31,32 Of 67 lamivudine-resistant Korean patients treated with ADV for 2 years, 9 (25.4%) showed ADV mutations.30 Eight of these nine patients had liver cirrhosis at baseline, including four with decompensated cirrhosis. Meanwhile, given that the recent data, most would agree that the standard of care for lamivudine resistance in HBV would be the addition of a nucleotide (ex. lamivudine plus ADV or emtricitabine plus tenofovir) rather than the substitution of a nucleotide, particularly in a decompensated patient in light of recent "consensus" recommendations.3 In recent study, ADV resistant rate at 3 years in patients with ADV added to lamivudine was 0% compared to 20% in patients switching to ADV monotherapy.33 However, this is retrospective study and the consensus was not solid in that time. In this study, 38 of 119 (31.9%) patients showed viral breakthrough during a median 33 months of ADV treatment, although genotypic ADV resistance was not tested in all patients. The viral breakthrough rate was relatively high because follow-up period was long (33 months) and concomitant lamivudine treatment was discontinued in most cases. In addition, even in patients with continued lamivudine treatment, the duration of combination treatment was too short for the proper evaluation of virologic outcome.
This study had some limitations. Its retrospective, observational design may lead to possible bias. Moreover, there was few with combination therapy in which patients were treated with both ADV and lamivudine. In addition, genotypic mutations conferring resistance to ADV were not analyzed. Despite these limitations, our study population clearly reflects the population of patients with lamivudine-resistant HBV receiving ADV rescue therapy for decompensation in South Korea, an area highly endemic for HBV. Although the clinical outcomes of HBV-related decompensated were serious, there was no evidence to support an alternative treatment strategy in decompensated patients with lamivudine-resistant HBV. In addition, this cohort study was large number, long-term follow up study compared to previous studies for decompensated patients with lamivudine resistant HBV.18,19
In conclusion, long term ADV treatment was effective and safe in decompensated patients with lamivudine-resistant HBV with resulted of ≥2-point decrease in CP score in 72.3% and a virological response rate of 56.3%. However, considering relatively high rates of early mortality and poor clinical outcome in suboptimal responder as well as viral breakthrough rate of 31.9% over a median follow-up period of 33 months, combination treatment with more potent, less resistant nucleos(t)ide would be better strategy in these patients. This needs to be validated in further prospective studies with longer-term follow-up periods.
Acknowledgements
This work was supported by clinical research grant from Pusan National University Hospital 2014.
REFERENCES
1. Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004;11:97-107. 14996343.


2. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 1988;61:1942-1956. 2834034.


4. Villeneuve JP, Condreay LD, Willems B, Pomier-Layrargues G, Fenyves D, Bilodeau M, et al. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology 2000;31:207-210. 10613747.


5. Kapoor D, Guptan RC, Wakil SM, Kazim SN, Kaul R, Agarwal SR, et al. Beneficial effects of lamivudine in hepatitis B virus-related decompensated cirrhosis. J Hepatol 2000;33:308-312. 10952249.


6. Perrillo R, Tamburro C, Regenstein F, Balart L, Bodenheimer H, Silva M, et al. Low-dose, titratable interferon alfa in decompensated liver disease caused by chronic infection with hepatitis B virus. Gastroenterology 1995;109:908-916. 7657121.


7. Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, et al. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology 2001;33:424-432. 11172345.


8. Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology 2002;123:719-727. 12198698.


9. Yao FY, Bass NM. Lamivudine treatment in patients with severely decompensated cirrhosis due to replicating hepatitis B infection. J Hepatol 2000;33:301-307. 10952248.


10. Bain VG, Kneteman NM, Ma MM, Gutfreund K, Shapiro JA, Fischer K, et al. Efficacy of lamivudine in chronic hepatitis B patients with active viral replication and decompensated cirrhosis undergoing liver transplantation. Transplantation 1996;62:1456-1462. 8958272.


11. Bae SH, Yoon SK, Choi JY, Jang JW, Cho SH, Yang JM, et al. Timing of lamivudine administration according to Child class in patients with decompensated cirrhosis. J Gastroenterol Hepatol 2005;20:1527-1532. 16174069.


12. Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. Asia Hepatitis Lamivudine Study Group. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology 2000;119:172-180. 10889166.


13. Locarnini S. Molecular virology and the development of resistant mutants: implications for therapy. Semin Liver Dis 2005;25(Suppl 1):9-19. 16103977.



14. Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003;348:808-816. 12606735.


15. Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2004;126:91-101. 14699491.


16. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003;348:800-807. 12606734.


17. Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology 2004;126:81-90. 14699490.


18. Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, et al. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology 2003;38:1419-1427. 14647053.


19. Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl 2007;13:349-360. 17326221.


20. Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol 2006;4:936-962. 16844425.


21. Allen MI, Gauthier J, DesLauriers M, Bourne EJ, Carrick KM, Baldanti F, et al. Two sensitive PCR-based methods for detection of hepatitis B virus variants associated with reduced susceptibility to lamivudine. J Clin Microbiol 1999;37:3338-3347. 10488202.



22. de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology 1992;103:1630-1635. 1426884.


23. Osborn MK, Han SH, Regev A, Bzowej NH, Ishitani MB, Tran TT, et al. Outcomes of patients with hepatitis B who developed antiviral resistance while on the liver transplant waiting list. Clin Gastroenterol Hepatol 2007;5:1454-1461. 17977800.



24. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-1531. 15470215.


25. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-871. 10733541.


26. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-470. 11172350.


27. Tseng PL, Lu SN, Tung HD, Wang JH, Changchien CS, Lee CM. Determinants of early mortality and benefits of lamivudine therapy in patients with hepatitis B virus-related decompensated liver cirrhosis. J Viral Hepat 2005;12:386-392. 15985009.


28. van Bömmel F, Wünsche T, Mauss S, Reinke P, Bergk A, Schürmann D, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology 2004;40:1421-1425. 15565615.


29. van Bömmel F, Zöllner B, Sarrazin C, Spengler U, Hüppe D, Möller B, et al. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology 2006;44:318-325. 16871563.


30. Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut 2006;55:1488-1495. 16461777.



31. Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006;43:1385-1391. 16729316.


Figure 1
Flow chart of patients. n, number; HCC, hepatocellular carcinoma, LT, liver transplantation; Tx, treatment; ADV, adefovir dipivoxil; HBV, hepatitis B virus.
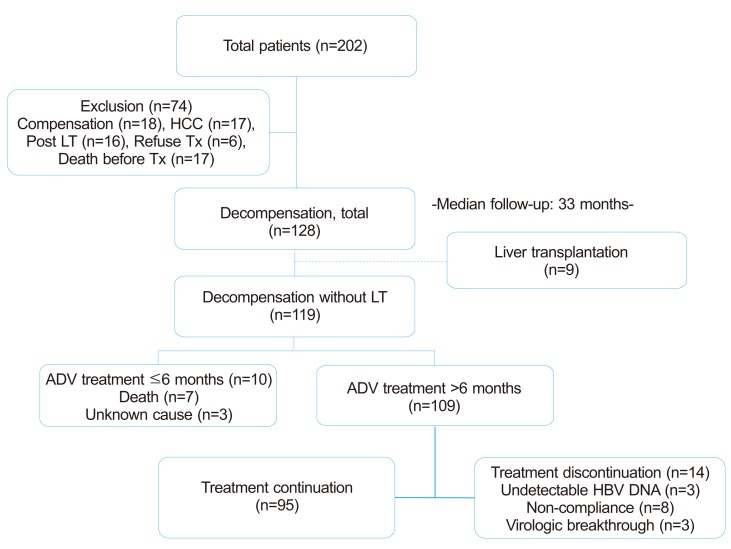
Figure 2
Change in Child-Pugh class with ADV rescue therapy. (A) Pretreatment Child-Pugh class B; (B) pretreatment Child-Pugh class C.

Figure 3
Cumulative survival curve of the 119 decompensated patients infected with lamivudine-resistant HBV who received ADV rescue treatment (median treatment duration, 33 months).

Figure 4
Cumulative survival curves of patients with sustained virologic suppression (n=67), virologic breakthrough (n=38), and suboptimal response (n=9) who received ADV rescue therapy. Patients with a suboptimal response to ADV treatment exhibited a significantly poorer overall survival rate than those with sustained virologic suppression or with virologic breakthrough (66.7% vs. 98% and 94.7%, respectively; P=0.012).
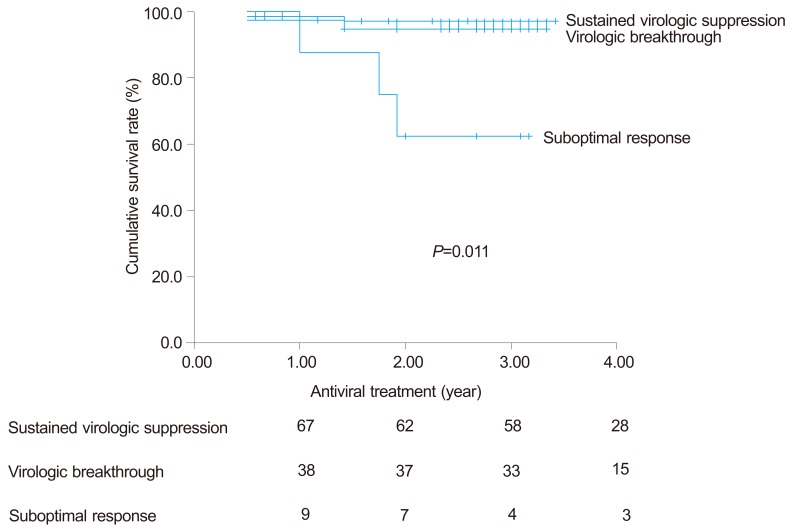
Table 1.
Baseline characteristics at the time of ADV treatment
Table 2.
Factors associated with Child-Pugh score improvement (≥2 points) following ADV treatment
| Baseline Variables | CP improvement (n=86) | Without CP improvement (n=23)* |
Univariateanalysis |
Multivariate analysis |
|
|---|---|---|---|---|---|
| P value† | P value† | OR (95% Confidence interval) | |||
| Age (years) | 50 (23-71) | 51 (38-65) | 0.268 | ||
| LAM duration (months) | 32 (6-67) | 40 (8-109) | 0.100 | 0.418 | 1.009 (0.988-1.030) |
| Overlap period (months) | 1 (0-25) | 2 (0-19) | 0.959 | ||
| Concomitant LAM Tx., n (%) | 0.477 | ||||
| Switch without overlapping | 40 (46.5) | 8 (34.8) | |||
| Switch with overlapping | 0 (0) | 4 (17.4) | |||
| Add-on | 46 (53.5) | 11 (47.8) | |||
| Serum HBV DNA (pg/mL) | 241.5 (0.5-6000) | 90.8 (2.9-6000) | 0.465 | ||
| ALT (IU/L) | 145 (32-2561) | 99 (28-820) | 0.081 | 0.550 | 0.999 (0.996-1.002) |
| Total bilirubin (mg/dL) | 3 (0.7-22.4) | 3.3 (0.9-29.3) | 0.504 | ||
| Albumin (g/L) | 3.0 (1.7-4.5) | 2.6 (2.1-4.3) | 0.040 | 0.903 | 1.073 (0.348-3.305) |
| Serum creatinine (mg/dL) | 0.9 (0.6-1.5) | 0.8 (0.5-11.8) | 0.002 | 0.118 | 1.310 (0.934-1.836) |
| Prothrombin time (INR) | 1.48 (1.07-3.38) | 1.62 (1.18-6.23) | 0.001 | 0.181 | 1.887 (0.744-4.789) |
| MELD | 15 (9-28) | 17 (9-37) | 0.001 | 0.506 | 0.932 (0.757-1.147) |
| Child-Pugh score | 9 (7-13) | 10 (7-13) | 0.003 | 0.427 | 1.242 (0.728-2.118) |
| Virologic response, n (%) | 0.001 | 0.001 | 3.984 (2.262-7.017) | ||
| Sustained virologic suppression | 55 (63.9) | 6 (26.1) | |||
| Virologic breakthrough | 29 (33.7) | 9 (39.1) | |||
| Suboptimal response | 2 (2.4) | 6 (26.1) | |||
Table 3.
Comparison of baseline characteristics according to the virologic response to ADV treatment
| Sustained virologic suppression (n=67) | Virologic breakthrough (n=38) | Suboptimal response (n=9) | P value† | |
|---|---|---|---|---|
| Age (years) | 50 (23-66) | 50 (27-71) | 51 (38-55) | 0.999 |
| LAM duration (months) | 37 (8-67) | 29 (6-109) | 37 (8-52) | 0.664 |
| Overlap period (months) | 1 (0-25) | 2 (0-19) | 10 (0-17) | 0.326 |
| Concomitant LAM Tx., n (%) | 0.852 | |||
| Switch without overlapping | 28 (41.8) | 16 (42.1) | 3 (33.3) | |
| Switch with overlapping | 36 (53.7) | 21 (55.3) | 5 (55.6) | |
| Add-on | 3 (4.5) | 1 (2.6) | 1 (11.1) | |
| Serum HBV DNA (pg/mL) | 236 (0.5-600) | 156.5 (3.3-2752.3) | 495 (61-3862.6) | 0.596 |
| ALT (IU/L) | 117 (28-1680) | 240 (49-2561) | 64 (52-126) | 0.002 |
| Total bilirubin (mg/dL) | 2.7 (0.7-20.80) | 3.4 (0.6-22.4) | 2.5 (0.7-4.1) | 0.100 |
| Albumin (g/L) | 3.1 (1.8-4.5) | 2.9 (1.7-4.3) | 2.8 (2.2-4.3) | 0.078 |
| Serum creatinine (mg/dL) | 0.90 (0.60-1.50) | 0.9 (0.6-9.8) | 0.8 (0.57-1.6) | 0.310 |
| Prothrombin time (INR) | 1.48 (1.07-3.38) | 1.47 (1.12-2.51) | 1.49 (1.35-2.17) | 0.591 |
| MELD | 14.5 (9-27) | 17 (10-28) | 14 (10-25) | 0.112 |
| Child-Pugh score, n (%) | 8 (7-13) | 9 (7-13) | 10 (7-12) | 0.162 |
| Class B | 49 (73.1) | 21 (55.3) | 4 (44.4) | 0.074 |
| Class C | 18 (26.9) | 17 (44.7) | 5 (55.6) |
Table 4.
Causes of death in 12 patients who died during ADV treatment



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




