INTRODUCTION
Hepatitis B virus (HBV) infection is prevalent in Asia, Africa, Southern Europe and Latin America, where the prevalence of HBsAg in the general population ranges from 2% to 20%.1 The long-term outcomes of chronic HBV infection vary widely. Literature review indicates that two-thirds of Asian HBV carriers have favorable clinical outcomes including inactive carrier state and loss of hepatitis B surface antigen (HBsAg), whereas one-third of them develop adverse outcomes including hepatic decompensation, cirrhosis and even hepatocellular carcinoma (HCC) in their lifetime.2
Because the lifetime risk of Asian HBV carriers to develop end-stage liver disease is as high as 15% to 40%.3 The identification of risk factors for the development of advanced liver disease, especially cirrhosis and HCC, in Asian HBV carriers is thus important for the implementation of effective antiviral treatment as early as possible. Recently, several qualitative and quantitative hepatitis B viral factors affecting the natural course and treatment outcome of HBV carriers have been identified.4,5 Among these viral factors, baseline serum HBV-DNA level is the main driving force for cirrhosis and HCC development in adult HBV carriers.6 Recently, quantitative hepatitis B surface antigen (qHBsAg) has been increasingly recognized to be a biomarker to predict both favorable and adverse outcomes of HBV carriers.7,8 In this review, qualitative and quantitative HBV factors affecting disease progression and the risk stratification for disease progression in Asian HBV carriers will be discussed.
Qualitative HBV factors
HBV genotype
At least 10 HBV genotypes (A to J) have been defined, and HBV genotype has been documented to have specific geographic distribution and influence the long-term outcomes of HBV infection.9 In Asia-Pacific region, HBV genotype B and C are the predominant strains and HBV genotype C patients, compared to genotype B patients, have late or absent HBeAg seroconversion after multiple hepatitis flares that accelerate the progression of chronic hepatitis.10,11 Most previous studies indicated that patients with HBV genotype C infection have a higher risk of cirrhosis and HCC than those with genotype B infection.9,12,13
HBV mutant
Mutations in precore, core promoter and deletion mutation in pre-S/S genes have been reported to be associated with the progression of liver disease, including cirrhosis and HCC.2 Previous studies revealed that dual mutations in basal core promoter (BCP) A1762T/G1764A were strongly associated with the risk of HCC development.13,14,15,16,17,18 Deletion mutations in the pre-S gene of HBV genome frequently occur in chronic HBV infection.2 The deletion over pre-S gene may cause accumulation of large surface protein in the endoplasmic reticulum (ER), resulting in ER stress and hepatocarcinogenesis.19,20,21
Quantitative HBV factors
During HBV replication, pregenomic RNA can be transcribed from covalently closed circular DNA (cccDNA) to serve as the template of negative-strand DNA through reverse transcription, and then fully double-stranded DNA through DNA polymerase within the nucleocapsid, finally with the assembly of envelope protein to form mature HBV virions.2 Several mRNAs can also be transcribed from cccDNA and then translate to viral proteins. Therefore, several qualitative viral factors can be clinically useful, including HBV-DNA from the infectious particles and circulating viral proteins such as HBsAg and HBeAg.
HBV-DNA level
Several commercial real-time PCR assays have been developed to detect and quantify HBV DNA.22 Nowadays, quantification of serum HBV-DNA is recommended by several international HBV guidelines to diagnose HBV infection, establish the indication for therapy, and to monitor antiviral treatment responses and emergence of drug resistance.23 HBV-DNA quantification also provides valuable prognostic information. The impact of viral load on the risk of HCC was assessed in a population-based prospective cohort of untreated CHB Taiwanese patients (REVEAL-HBV study).24 The data showed that the cumulative incidence of HCC increased with serum HBV DNA level. It ranged from 1.3% to 14.9% for patients with an HBV DNA level of less than 300 copies/ml (~60 IU/ mL) and 106 copies/ml (~200,000 IU/mL) or more, respectively (P<0.001). A recent hospital-based cohort study further validated the HCC risk started to increase when HBV-DNA level was higher than 2,000 IU/mL.25 All these data confirm that HBV-DNA level is the trigger of hepatitis activity and the strongest risk factor associated with HCC development in adult Asian patients with chronic HBV infection.
HBsAg quantification
In addition to HBV-DNA level, the clinical significance of quantitative HBsAg has become increasingly recognized.7 This old biomarker has a new role in current management of chronic HBV infection. In the natural course of HBV infection, HBV-DNA is only from infectious particles and levels reflect viral replication. Accordingly, a decline of HBV-DNA means reduction of HBV replication. In contrast, HBsAg can be derived from both mature virions and defective particles. Thus serum HBsAg level not only reflects the cccDNA transcription or mRNA translation, but also host immune control over HBV infection.26,27 Our recent study showed that low serum HBsAg level (<100 IU/mL), alone or in combination with HBV-DNA levels, at 1 year after HBeAg seroconversion could predict HBsAg loss in patients with HBV genotype B or C infection.28 In addition, qHBsAg was better than serum HBV-DNA level for the prediction of spontaneous HBsAg loss in HBeAg-negative carriers with a low viral load (<2,000 IU/mL). Moreover, HBsAg level <10 IU/mL was the strongest predictor of HBsAg loss in patients with a low viral load.29
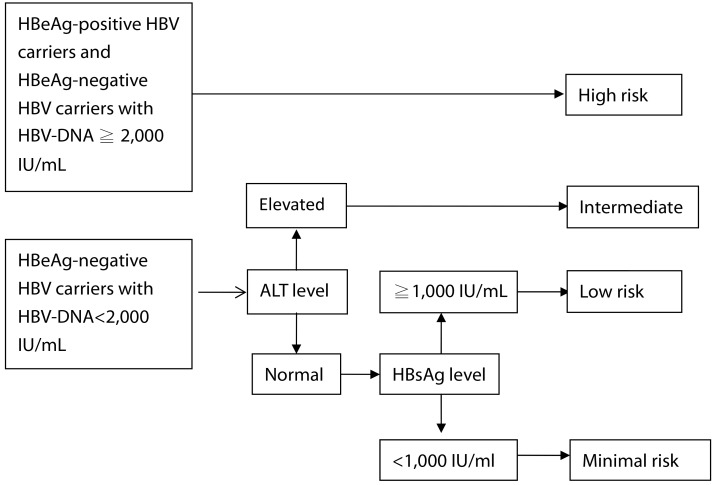
Taken together, there indeed exists a correlation between qHB-sAg and liver disease progression. In the recent update of REVEAL-HBV study, qHBsAg was analyzed, and the results showed that both HBsAg and HBV-DNA levels are independent predictors of HCC development.30 Our hospital-based ERADICATE-B study (Elucidation of Risk Factors for Disease Control or Advancement in Taiwanese Hepatitis B Carriers) also showed similar findings. In brief, a total of 2,688 non-cirrhotic Taiwanese chronic hepatitis B patients followed for a mean of 14.7 years. HCC risk increased when patients had increased HBV DNA level.31 Although the incidence of HCC is significantly associated with baseline serum HBV-DNA levels, inactive or low risk HBV carriers (serum HBV-DNA levels <2,000 IU/ml) still have significantly higher hazard ratios for HCC compared with individuals without HBV infection. Therefore, it is important to identify factors predictive of HCC other than HBV-DNA level in these inactive or low risk HBV carriers. In the ERADICATE-B study, we evaluated 1,068 HBeAg-negative patients with low viral load (<2,000 IU/mL). Risk factors for HBeAg-negative hepatitis as well as HCC development included advanced age, male gender, elevated levels of ALT and high qHBsAg (≧1,000 IU/mL), but not levels of HBV DNA. Multivariate analysis revealed that qHBsAg ≧1,000 IU/mL was an independent risk factor for HCC development.31 Data from REVEAL-HBV study and ERADICATE-B study all showed that serum HBsAg and HBV DNA levels were complementary markers in predicting disease progression to cirrhosis and HCC.24,31,32
Risk calculator and algorithm for disease progression
Several host and viral factors predictive of HCC risk have been identified, and the REACH-B (Risk Estimation for Hepatocellular Carcinoma in Chronic Hepatitis B) study has developed and validated a predictive score for the risk of development of HCC in patients with chronic hepatitis B.33 This study included risk score development cohort with 3,584 non-cirrhotic CHB Taiwanese and a validation cohort with 1,050 patients from three independent hospitals of Hong Kong and Republic of Korea. The 17-point risk score is composed of 5 predictors of HCC, including sex, age, serum ALT level, HBeAg status, and serum HBV-DNA level. The risk score could precisely estimate the risk of HCC development at 3, 5, and 10 years of follow-up. Nevertheless, this risk scoring system of HCC may underestimate risk for patients with very low viral load at baseline. In ERADICATE-B study, the risk of HCC for carriers with HBV-DNA <2,000 IU/mL and HBsAg ≧1,000 IU/mL was much higher than those with HBV-DNA <2,000 IU/mL and HBsAg <1,000 IU/mL.31 This finding indicated that high HBsAg level itself may reflect inadequate host immune control against HBV infection, leading to an increased HCC risk over time in patients with low viral load. Therefore, serum HBsAg level should be integrated into the HCC risk calculator for future management of patients with chronic HBV infection, particularly in those with low and intermediate viral loads. Furthermore, the relationships between HCC risk and dynamic changes of serum HBV-DNA, HBsAg, and ALT levels were determined in the ERADICATE-B study. Compared to patients with persistently low levels of HBV-DNA, HBsAg, or ALT, those with persistently high levels of these 3 factors were at a higher risk of HCC.32 Therefore, it is essential to incorporate qualitative and quantitative hepatitis B viral factors in risk calculation model to make it more comprehensive and to be clinically useful in various forms of chronic liver disease, including inactive carrier state, chronic hepatitis and cirrhosis (Fig.).
Transient elastography-based risk estimation
The severity of hepatic fibrosis is known to be associated with HCC risk,34 however, this parameter has not been incorporated in the risk model for HCC development because of the invasive nature of liver biopsy. Recently, liver stiffness measurement by using transient elastography has been introduced as a noninvasive method for assessing the severity of hepatic fibrosis.35 A previous study showed that the baseline liver stiffness values could be an independent predictor of HCC in patients with chronic HBV infection, where the 3-year cumulative incidence of HCC was significantly higher in patients with a higher liver stiffness value.34
A recent Korean study included 1,250 CHB patients with baseline liver stiffness values to construct a predictive model for HCC occurrence based on a Cox proportional hazards model.36 By using multivariate analysis, age, male gender, and liver stiffness values were independent predictors of HCC, whereas HBV-DNA level ≥20,000 IU/L showed borderline statistical significance. A predictive model for HCC was developed using these 4 variables, with a correlation coefficient of 0.905 between predicted risk and observed risk of HCC occurrence. The suggested formula for 3-year probability of HCC occurrence is 1 - PA (A=exp [0.05306×age+1.106×male gender+0.04858×liver stiffness value+0.50969×HBV DNA ≥20,000 IU/L]). Thus, this novel model may accurately estimate the risk of HCC occurrence in Asian patients with chronic HBV infection.
CONCLUSIONS
Several host and HBV factors such as age, gender, ALT level, hepatic fibrosis stage, serum HBsAg level, viral load, genotype and mutants are shown to affect disease progression and HCC development in chronic hepatitis B patients. On the basis of population and hospital-based cohort studies, risk stratification for disease progression and HCC in patients with chronic HBV infection has been established step by step. In the future, multivariate risk assessment profiles for disease progression and HCC should be integrated with current HBV treatment guidelines to enable practicing physicians to have better management of HBV carriers with different HCC risks.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




