| Clin Mol Hepatol > Volume 20(3); 2014 > Article |
ABSTRACT
Prediction of liver fibrosis progression has a key role in the management of chronic viral hepatitis, as it will be translated into the future risk of cirrhosis and its various complications including hepatocellular carcinoma. Both hepatitis B and C viruses mainly lead to fibrogenesis induced by chronic inflammation and a continuous wound healing response. At the same time direct and indirect profibrogenic responses are also elicited by the viral infection. There are a handful of well-established risk factors for fibrosis progression including older age, male gender, alcohol use, high viral load and co-infection with other viruses. Metabolic syndrome is an evolving risk factor of fibrosis progression. The new notion of regression of advanced fibrosis or even cirrhosis is now strongly supported various clinical studies. Even liver biopsy retains its important role in the assessment of fibrosis progression, various non-invasive assessments have been adopted widely because of their non-invasiveness, which facilitates serial applications in large cohorts of subjects. Transient elastography is one of the most validated tools which has both diagnostic and prognostic role. As there is no single perfect test for liver fibrosis assessment, algorithms combining the most validated noninvasive methods should be considered as initial screening tools.
Liver fibrosis represents a pervasive wound-healing response, driven primarily by the development of inflammation in response to parenchymal acute or chronic injury.1 Liver fibrosis may be represented by variable clinical manifestations, which are determined by the type and extent of liver damage, the underlying liver disease and the capacity of the whole body to respond. Cirrhosis, the end stage of liver fibrosis, is characterized by architectural disruption, aberrant hepatocyte regeneration, nodule formation and vascular changes.2 Cirrhosis substantially increases the risk of hepatocellular carcinoma, cirrhotic complications and death.3,4 Therefore it is important to accurately predict the rate of liver fibrosis progression in patients with chronic viral hepatitis, which has important clinical significance in terms of prognostic and treatment implications. On the other hand, with effective antiviral agents that potentially reverse liver fibrosis,5,6 methods of assessing fibrosis is essential to monitor disease progression, clinical outcomes, and response to treatment are warranted.
There is no doubt that the risks of various complications increase dramatically if patients reached the stage of cirrhosis.7 In fact the risk starts to increase as early as F2 fibrosis. In a cohort of 188 Korean CHB patients followed up for nearly 10 years, the cumulative probability of developing cirrhosis for patients with Metavir stage F0 or F1 fibrosis at baseline was 0%, 11% and 11% at 5 years, 10 years and 15 years respectively. The probabilities were increased significantly with baseline fibrosis at stage F2 (12%, 33% and 47% at 5 years, 10 years and 15 years respectively) and stage F3 (22%, 47% and 65% at 5 years, 10 years and 15 years respectively).8 In another cohort of 2,215 patients with chronic viral hepatitis, the relative risk of developing cirrhosis and HCC in patients with F3 fibrosis was 6.3 (95% confidence interval [CI] 3.9-10.1) and 4.4 (95% CI, 2.4-7.8) compared with those with F1 and F2 fibrosis.9
The presence of HBV and HCV mainly lead to fibrogenesis induced by chronic inflammation and a continuous wound healing response. Additionally, direct and indirect profibrogenic responses are also elicited by the viral infection. HBV X protein induces paracrine activation of human hepatic stellate cells (HSCs).10 In vitro experiments indicate that HBV affects the proliferation and expression of collagen I in HSCs.11 Hepatitis B e antigen (HBeAg) was recently found to induce the activation and proliferation of HSCs, mainly mediated by transforming growth factor beta (TGF-β), and HBeAg protein purified from cell medium can directly activate HSCs.12
Several earlier studies described the pro-apoptotic, steatosis-inducing and cancerogenic effects of HCV core protein and nonstructural protein 5A (NS5A).13 Direct profibrogenic effects of HCV are demonstrated in HuH7 hepatoma cells which propagate the NS3-NS5 replicon, release profibrogenic factors, mainly active TGF-β1 that induces profibrogenic and suppresses fibrolytic genes and proteins in HSC and myofibroblasts.14 HCVE2 protein has been implicated in fibrogenesis, since it induces profibrogenic matrix metalloproteinase (MMP)-2 in HSC.15
Chronic hepatitis B (CHB) patients may have variable disease course with outcomes ranging from inactive carrier state, active hepatitis, liver fibrosis to cirrhosis.16 The well-established risk factors for progression from mild to advanced liver fibrosis in CHB include older age, male gender, alcohol use, co-infection with hepatitis C virus, hepatitis D virus, or human immunodeficiency virus, elevated alanine aminotransferase (ALT) levels, and high hepatitis B virus (HBV) DNA level (Table 1).17 Ongoing HBV replication or presence of HBeAg may accelerate the progression of chronic hepatitis to advanced fibrosis cirrhosis.18 Delayed HBeAg seroconversion (over 40 years of age) and HBeAg seroreversion after spontaneous HBeAg seroconversion, indicating a prolonged period of viral replication and necroinflammation, were associated with increased risk of cirrhosis.19 Genotype C HBV was associated with more severe liver fibrosis than genotype B HBV, probably because of delayed HBeAg seroconversion and prolonged active disease.20
Two recent studies made use of serial liver stiffness measurements (LSM) with transient elastography to assess the change in liver fibrosis in large cohorts of asymptomatic CHB patients. Liver fibrosis progression was defined as an increase in LSM by 30% or more.21,22 The study of 361 patients with inactive HBeAg-negative CHB demonstrated that liver fibrosis progression is rare in if their serum HBV DNA <20,000 IU/ml and normal ALT.21 Nonetheless around 24.8% of patients with HBV DNA between 2,000IU/ml and 20,000 IU/ml developed treatment indications during follow-up.21 The study of 247 HBeAg-positive CHB patients revealed that 4.1% and 6.6% patients in immune-tolerant and immune-reactive phase had liver fibrosis progression, whereas and 12.2% and 67.2% of them received antiviral therapy respectively.22 These observations provided indirect evidence that antiviral therapy aborts liver fibrosis progression.
Studies conducted before the introduction of the highly active anti-retroviral therapy (HAART) have shown that human immunodeficiency virus (HIV)-related immune deficiency modifies the natural history of CHB with higher levels of HBV replication and a lower rate of spontaneous HBeAg seroconversion, leading to a more rapid liver fibrosis progression towards cirrhosis.23 Chronic HDV infection leads to more severe liver disease than chronic HBV mono-infection with an accelerated course of fibrosis progression, an increased risk of hepatocellular carcinoma and early decompensation in the setting of established cirrhosis.24 Recipients with graft occult HBV infection (O-HBV) and no O-HBV in the native liver who received their grafts from donors aged above 40 years.25
Recent data from Chinese and Korean cohorts established that metabolic syndrome is a risk factor of advanced liver fibrosis and cirrhosis independent of viral factors in CHB.26,27 In a recent prospective cohort study of 663 CHB patients, new-onset metabolic syndrome and some of its components (namely central obesity and low high-density lipoprotein cholesterol) were found associated with liver fibrosis progression (Fig.).28 Even the effect of such coincident metabolic syndrome was most apparent in the immune tolerant phase; its effect was independent of change in viral load and ALT level.28 This is supported by the observation from a survey in general population that CHB is associated with a lower prevalence of fatty liver, hypertriglyceridemia and metabolic syndrome.29
Chronic hepatitis C (CHC) is associated with variable rates of fibrosis progression. Established cofactors for fibrosis progression include older age at infection, male gender, chronic alcohol consumption, obesity, insulin resistance and type 2 diabetes, and immunosuppression therapy.30 In spite of slow disease progression over the initial 20 years of infection, advancing age may accelerate fibrosis progression.31 Recent studies suggest an association between hepatitis C virus (HCV) genotype 3 and accelerated liver disease progression.32
After liver transplantation, fibrosis progression is highly accelerated in patients with recurrent hepatitis C with development of bridging fibrosis and cirrhosis in 20-54% at 5 years and 32-51% at 7 years.33 HIV co-infection is an important contributor to progression of chronic hepatitis C. HIV envelope protein gp120 blocks insulin signaling and via induction of oxidative stress increases profibrogenic tissue inhibitor of metalloproteinase-1 (TIMP-1) in HCV-replicon cells, and also directly induces procollagen synthesis in cultured hepatic stellate cells (HSC).34 The more rapid liver fibrosis progression to F4 was observed for men with alcoholic fatty liver disease, and the slower for women with non-alcoholic fatty liver disease (NAFLD).35
There is ample evidence to support the fact that effective antiviral therapy potentially reverses liver fibrosis in CHB patients.6,36 The first solid evidence came from a cohort of 57 patients who received at least 3 years of cumulative entecavir therapy, among whom 88% had ≥1-point improvement in the Ishak fibrosis score, including all 10 patients with advanced fibrosis or cirrhosis.36 This notion was further consolidated by a larger cohort of 348 patients who tenofovir disoproxil fumarate, in which 176 (51%) had regression of fibrosis at week 240.6 More impressively, of the 96 (28%) patients with cirrhosis (Ishak score 5 or 6) at baseline, 71 (74%) had regression of cirrhosis (≥1 unit decrease in the score). Data from the same trial revealed that steatosis at baseline correlated with decreased regression of cirrhosis at week 240; 73% of ≥grade 1 patients had regression of cirrhosis vs. 88% of grade 0 patients; nonetheless body mass index remained the only significant factor in multivariate analysis.37
Data has been available since early this century to illustrate the fact that interferon therapy regress liver fibrosis in CHC patients with SVR.38 Similar findings have been reported in sustained responders to pegylated interferon.39,40 Regression of liver fibrosis, which occurred in 82% of patients, was sustained at 5 years after SVR; more impressively recovery of normal or nearly normal liver architecture is possible.5
It is a big challenge to use interferon-based antiviral therapy in post-transplantation recurrent HCV infection because of its side effects and concern on triggering rejection.41 Therefore its effects on fibrosis progression have been little investigated. Antiviral therapy is commonly started when fibrosis reaches F2 or above; stabilization or even improvement of fibrosis may be seen in patients with or without sustained virologic response (SVR).42,43 The data from a randomized controlled illustrated that antiviral therapy slows fibrosis progression provided it is started early (F0-2).44
In the treatment guidelines issued by different authorities of liver diseases, severity of liver fibrosis, together with ALT and HBV DNA level, has a central and important role on the decision-making process of CHB.45,46,47 Improvement and even regression of liver fibrosis and cirrhosis following successful antiviral treatment are questioning the need for a follow-up liver biopsy.5 Longitudinal measurements using noninvasive methods could allow an effective monitoring of the dynamic changes of liver fibrosis, as shown in the liver transplant setting.48
Liver biopsy has been the gold standard of liver fibrosis assessment in the last few decades. However it is often challenged for its diagnostic accuracy limited by the sampling variability as the average size of biopsy is 15 mm in length, which only represents 1/50,000 the size of the entire liver.49 There is significant variability in the histologic assessment of two readings of the same biopsy by the same pathologist, and between two pathologists, even among those who are highly specialized.50 Furthermore, all of histologic scoring systems are discontinuous and hence semi-quantitative.51 Pain is reported by one-third of the patients undergone liver biopsy, whereas a severe complication (which is life-threatening or prolongs hospitalization) occurs in 3 out of 1000 cases and death is reported in 3 out of 10,000 cases.52 Liver biopsy may also lead to selection bias towards more active disease such that the incidence of advanced fibrosis would be overestimated.53 All these problems make it impractical to perform serial biopsies to assess disease progression in routine clinical practice.54
Reliable non-invasive methods for the assessment of liver fibrosis are increasingly being incorporated in the management of patients with chronic viral hepatitis, helping predict prognosis, guide treatment decisions, and stratify patients for antiviral therapy as well as emerging antifibrotic therapies.33
Transient elastography by Fibroscan (Echosens, Paris, France) has been developed as an accurate, reproducible and non-invasive test for the assessment of advanced liver fibrosis.7 It has been the most widely-validated tools in essentially all chronic liver diseases including CHB and CHC.55 The beauty of this tool is its non-invasiveness, which makes it possible to perform repeated liver fibrosis assessments on a large number of asymptomatic patients in a cross-sectional and serial fashion.21,22,56,57
The remaining controversies include the optimal cutoff values to diagnose advanced fibrosis and cirrhosis, which differ according to particular etiologies. The suggested diagnostic performance and cutoff values for histologic cirrhosis (F4) in CHB and CHC based on published studies are summarized in Table 2. Another issue is the definition of liver fibrosis progression based on LSM. One of the initial studies defined it an increase of LSM above 30%,58 mainly because an interquartile range (IQR) within 30% of LSM would reflect reliable results.59 Some investigators studied dynamic changes of LSM according to different strata of LSM.60,61
A 3rd generation transient elastography Fibrotouch (Wuxi Hisky Medical Technology Co Ltd, Beijing, China) has been available in clinical use since 2013. The potential advantage of Fibrotouch is it may overcome obesity, as the depth of measurement will be adjusted according the thickness subcutaneous fat in obese patients with the dynamic probe.62
Magnetic resonance elastography (MRE) is probably one of the best non-invasive assessments of liver fibrosis in terms of diagnostic accuracy for different stages of liver fibrosis. MRE can discriminate between patients with moderate or more fibrosis (≥F2).63 The technical success rate of magnetic resonance elastography was high (94%), and the AUROCs of MRE were also impressive (0.994 for ≥F2; 0.985 for ≥F3; 0.998 for ≥F4).64 Even so, the major limitation of MRE is its availability, as MR machine may not be readily available in some centers. The long examination time also poses implication on the cost of the examination.65
Acoustic radiation force impulse imaging (ARFI) involves mechanical excitation of tissue using short duration acoustic pulses that propagate shear waves and generate localized, µ-scale displacements in tissue.66 The major constraints of ARFI is its narrow range of values (0.5-4.4 meters/sec), in contrast to LSM by transient elastography (1.0-75.0 kPa). This may limit the definitions of cutoff values to define various stage of fibrosis.67 Shear wave elastography (SWE, SuperSonic Imagine, Aix-en-Provence, France) is an absolute quantification of tissue stiffness in terms of pressure unit of kiloPascal (kPa) rather than producing semi-quantitative estimate corresponding to relative tissue strain.68 SWE was found more accurate than Fibroscan in liver fibrosis ≥F2.69 All these imaging techniques were mostly assessed in a cross-sectional fashion such that accuracy on applying them in longitudinal fashion remains uncertain.
Serum markers for liver fibrosis are classified as direct (or class I) which represent extracellular matrix components (reflecting the pathophysiology of liver fibrogenesis); and indirect (or class II) which use routine laboratory data (reflecting the consequences of the liver damage). Direct and indirect markers may be used alone or, more commonly, in combination to produce composite scores.70 Serum markers generally have modest accuracy to diagnose advanced liver fibrosis.71 A non-invasive test independent of the serum ALT or AST levels may be a good supplementary test for LSM. Among various serum test formulae, Forns index72 and Hui index73 are composed of clinical parameters other than ALT or AST levels. We demonstrated that combined LSM-Forns or LSM-ELF algorithm improved the accuracy to predict advanced liver fibrosis in CHB patients.74,75
The Fibrotest (proprietary formula; Biopredictive, Paris, France) is the most widely validated indirect serum marker panel, most extensively studied in CHC.76 It is computed using five parameters, namely total bilirubin, haptoglobin, gamma-glutamyl-transpeptidase, a2-macroglobulin and apolipoprotein-A.77 In a systematic review including 9 studies (1,679 patients), an excellent discrimination was found for identifying cirrhosis, but a lesser ability to identify significant (≥F2) fibrosis.78 The combination of LSM and Fibrotest was found to have the best diagnostic performance compared to either test alone in patients with CHC.79
Serial monitoring of liver fibrosis with non-invasive tools becomes indispensable and is replacing serial liver biopsy examinations in clinical studies and practice. The issue left is that the validation of novel noninvasive tools is often based on liver biopsy, the imperfect gold standard of liver fibrosis assessment. Moreover, histological scores of liver fibrosis are ordinal categories with no quantitative relationship between them and therefore inappropriate to use as continuous variables.80 Noninvasive markers of liver fibrosis should be ideally validated against quantitative histological measures. In this setting, collagen proportionate area (CPA) defined as the proportion of the area of the biopsy occupied by collagen measured by computer-assisted morphometric analysis of digital images81 should be adopted to compare with noninvasive methods.82
As there is no single perfect test for liver fibrosis assessment, algorithms combining the most validated noninvasive methods, most likely one imaging-based and one serum-based marker, should be considered as initial screening tools. In cases of indeterminate or discordant results, liver biopsy can be performed to confirm the exact stage of fibrosis. Recently, the Asian Pacific Association for the Study of the Liver (APASL) consensus on liver fibrosis has recommended the use of a stepwise algorithm of using noninvasive markers, concluding that this approach may reduce the need for liver biopsy by 30%.83 Hence, integration of noninvasive assessments into clinical guidelines will become standard clinical practice shortly.
REFERENCES
1. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010;7:425-436. 20585339.



2. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425-456. 21073339.


3. Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis 2006;26:153-161. 16673293.



4. Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology 2013;144:933-944. 23415803.


5. George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009;49:729-738. 19072828.



6. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468-475. 23234725.


7. Wong GL. Transient elastography: Kill two birds with one stone? World J Hepatol 2013;5:264-274. 23717737.



8. Park BK, Park YN, Ahn SH, Lee KS, Chon CY, Moon YM, et al. Long-term outcome of chronic hepatitis B based on histological grade and stage. J Gastroenterol Hepatol 2007;22:383-388. 17295771.


9. Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol 1998;28:930-938. 9672166.


10. Martin-Vilchez S, Sanz-Cameno P, Rodriguez-Munoz Y, Majano PL, Molina-Jiménez F, López-Cabrera M, et al. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology 2008;47:1872-1883. 18449922.


11. Liu X, Zhu ST, You H, Cong M, Liu TH, Wang BE, et al. Hepatitis B virus infects hepatic stellate cells and affects their proliferation and expression of collagen type I. Chin Med J (Engl) 2009;122:1455-1461. 19567171.

12. Zan Y, Zhang Y, Tien P. Hepatitis B virus e antigen induces activation of rat hepatic stellate cells. Biochem Biophys Res Commun 2013;435:391-396. 23665329.


13. Schuppan D, Krebs A, Bauer M, Hahn EG. Hepatitis C and liver fibrosis. Cell Death Differ 2003;10(Suppl 1):S59-S67. 12655347.



14. Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, et al. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology 2005;129:246-258. 16012951.


15. Taniguchi H, Kato N, Otsuka M, Goto T, Yoshida H, Shiratori Y, et al. Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J Med Virol 2004;72:52-59. 14635011.


18. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678-686. 16530509.


19. Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004;116:829-834. 15178498.


20. Chan HL, Wong GL, Tse CH, Chim AM, Yiu KK, Chan HY, et al. Hepatitis B virus genotype C is associated with more severe liver fibrosis than genotype B. Clin Gastroenterol Hepatol 2009;7:1361-1366. 19683072.


21. Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol 2013;28:1842-1848. 23829381.


22. Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression in chronic hepatitis B patients positive for hepatitis B e antigen: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol 2013;28:1762-1769. 23808759.


23. Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006;44(Suppl):S65-S70. 16338021.


24. Alvarado-Mora MV, Locarnini S, Rizzetto M, Pinho JR. An update on HDV: virology, pathogenesis and treatment. Antivir Ther 2013;18:541-548. 23792471.


25. Toniutto P, Minisini R, Fabris C, De Feo T, Marangoni F, Burlone M, et al. Occult hepatitis B virus infection in liver transplant recipients with recurrent hepatitis C: relationship with donor age and fibrosis progression. Clin Transplant 2009;23:184-190. 19210526.


26. Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut 2009;58:111-117. 18832522.


27. Yoon H, Lee JG, Yoo JH, Son MS, Kim DY, Hwang SG, et al. Effects of metabolic syndrome on fibrosis in chronic viral hepatitis. Gut Liver 2013;7:469-474. 23898389.



28. Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther 2014;39:883-893. 24612251.


29. Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol 2012;56:533-540. 22027575.


30. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med 2011;364:2429-2438. 21696309.


31. Grebely J, Dore GJ. What is killing people with hepatitis C virus infection? Semin Liver Dis 2011;31:331-339. 22189973.



32. Probst A, Dang T, Bochud M, Egger M, Negro F, Bochud PY. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis. J Viral Hepat 2011;18:745-759. 21992794.


33. Berenguer M, Schuppan D. Progression of liver fibrosis in post-transplant hepatitis C: mechanisms, assessment and treatment. J Hepatol 2013;58:1028-1041. 23262248.


34. Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology 2008;134:803-811. 18325393.


35. Poynard T, Munteanu M, Deckmyn O, Ngo Y, Drane F, Castille JM, et al. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: proof of concept and first application in a large population. J Hepatol 2012;57:541-548. 22612998.


36. Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886-893. 20683932.


37. Buti M, Washington MK, Gane E, Aguilar Schall R, Bornstein JD, Subramanian M, et al. Hepatic steatosis does not predict regression of liver cirrhosis in chronic hepatitis B (CHB) patients treated with tenofovir disoproxil fumarate (TDF). [Abstract]. J Hepatol 2014;60(Suppl):S294-S295.

38. Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000;132:517-524. 10744587.


39. Camma C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004;39:333-342. 14767986.


40. Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002;122:1303-1313. 11984517.


41. De Martin E, Senzolo M, Gambato M, Germani G, Vitale A, Russo FR, et al. Fibrosis progression and the pros and cons of antiviral therapy for hepatitis C virus recurrence after liver transplantation: a review. Transplant Proc 2010;42:2223-2225. 20692449.


42. Bizollon T, Ahmed SN, Radenne S, Chevallier M, Chevallier P, Parvaz P, et al. Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut 2003;52:283-287. 12524414.



43. Bizollon T, Pradat P, Mabrut JY, Radenne S, Ducerf C, Baulieux J, et al. Histological benefit of retreatment by pegylated interferon alfa-2b and ribavirin in patients with recurrent hepatitis C virus infection posttransplantation. Am J Transplant 2007;7:448-453. 17173661.


44. Carrion JA, Navasa M, Garcia-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology 2007;132:1746-1756. 17484872.


45. European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-185. 22436845.


46. Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012;6:531-561.


48. Carrion JA, Torres F, Crespo G, Miquel R, García-Valdecasas JC, Navasa M, et al. Liver stiffness identifies two different patterns of fibrosis progression in patients with hepatitis C virus recurrence after liver transplantation. Hepatology 2010;51:23-34. 19839063.


49. Garcia-Tsao G, Boyer JL. Outpatient liver biopsy: how safe is it? Ann Intern Med 1993;118:150-153. 8416312.


50. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-1457. 14647056.


51. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-293. 8690394.


52. Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep 2004;6:30-36. 14720451.


54. Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010;59:969-974. 20581244.


55. Wong GL. Update of liver fibrosis and steatosis with transient elastography (Fibroscan). Gastroenterol Rep (Oxf) 2013;1:19-26. 24759663.




56. Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Evaluation of alanine transaminase and hepatitis B virus DNA to predict liver cirrhosis in hepatitis B e antigen-negative chronic hepatitis B using transient elastography. Am J Gastroenterol 2008;103:3071-3081. 19086958.


57. Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol 2009;7:227-233. 19121647.


58. Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. On-treatment monitoring of liver fibrosis with transient elastography in chronic hepatitis B patients. Antivir Ther 2011;16:165-172. 21447865.


59. Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007;56:968-973. 17255218.



60. Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011;53:885-894. 21319193.


61. Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 2012;57:556-563. 22617153.


62. Fibro Touch. Noninvasive liver fibrosis diagnostic M type:advantages. Fibro Touch web site. 2014.07.02.]. <http://www.fibrotouch.com/goods/productyoushi1.aspx>.
63. Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 2007;5:1207-1213.e2. 17916548.



64. Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135:32-40. 18471441.


65. Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544-555. 23423795.



66. Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol 2002;28:227-235. 11937286.


67. Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293-1302.e4. 22537436.


68. Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol 2009;35:219-229. 19081665.


69. Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology 2013;269:910-918. 23912619.


70. Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol 2012;25:218-231. 24714123.


71. Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006 2;43(2 Suppl 1):S113-S120. 16447288.


72. Forns X, Ampurdanes S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002 10;36(4 Pt 1):986-992. 12297848.


73. Hui AY, Chan HL, Wong VW, Liew CT, Chim AM, Chan FK, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol 2005;100:616-623. 15743360.


74. Wong GL, Chan HL, Choi PC, Chan AW, Yu Z, Lai JW, et al. Non-invasive algorithm of enhanced liver fibrosis and liver stiffness measurement with transient elastography for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2014;39:197-208. 24261924.


75. Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2010;31:1095-1103. 20180785.


76. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001;357:1069-1075. 11297957.


77. Rossi E, Adams L, Prins A, Bulsara M, de Boer B, Garas G, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003;49:450-454. 12600957.


78. Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007;102:2589-2600. 17850410.


79. Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343-350. 15685546.


80. Germani G, Burroughs AK, Dhillon AP. The relationship between liver disease stage and liver fibrosis: a tangled web. Histopathology 2010;57:773-784. 20812954.


81. Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, et al. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol 2008;6:1027-1035. 18456573.


82. Tsochatzis EA, Germani G, Dhillon AP, Burroughs AK. Noninvasive assessment of liver fibrosis: the clinical context and question are important. Hepatology 2011;54:2276. 21793024.

83. Shiha G, Sarin SK, Ibrahim AE, Omata M, Kumar A, Lesmana LA, et al. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009;3:323-333. 19669358.



84. Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat 2009;16:36-44. 18673426.


85. Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 2009;29:242-247. 18637064.


86. Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 2007;14:360-369. 17439526.


87. Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005;41:48-54. 15690481.


88. Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008;47:380-384. 18095306.


89. de Ledinghen V, Ratziu V, Causse X, Le Bail B, Capron D, Renou C, et al. Diagnostic and predictive factors of significant liver fibrosis and minimal lesions in patients with persistent unexplained elevated transaminases. A prospective multicenter study. J Hepatol 2006;45:592-599. 16837098.


90. Vergara S, Macias J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D, et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis 2007;45:969-974. 17879910.



Figure
Adjusted odds ratio (adjusted for the change in serum alanine aminotransferase and hepatitis B virus DNA levels) of new-onset metabolic syndrome and its factors for liver fibrosis progression and regression.28 HDL-C, high density lipoprotein cholesterol; TG, triglycerides.
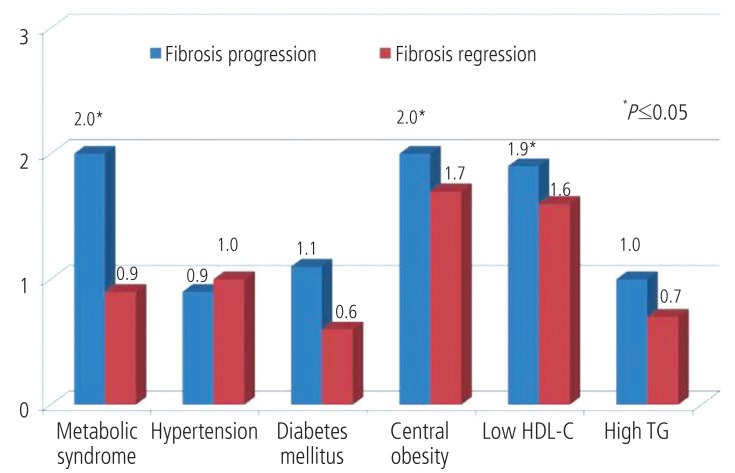
Table 1.
Factors associated with increased risk of progression to cirrhosis
Table 2.
Diagnostic performance and suggested cutoff values of liver stiffness measurement for the diagnosis of histologic cirrhosis7
| Authors | Chan et al. 2009 [84] | Wong et al. 2010* [75] | Marcellin et al. 2009 [85] | Coco et al. 2007 [86] | Ziol et al. 2005 [87] | Castera et al. 2005 [79] | Arena et al. 2008 [88] | de Ledinghen et al. 2006 [89] | Vergara et al. 2007 [90] | Rigamonti et al. 2008 [91] | Carrion et al. 2006 [92] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of biopsies | 161 | 238 | 173 | 228 | 251 | 183 | 150 | 72 | 169 | 95 | 124 |
| Prevalence of cirrhosis (%) | 25.0 | 23.5 | 8.0 | 20.2 | 19.0 | 25.0 | 19.3 | 23.6 | 38.5 | 17.0 | 11.0 |
| Etiologies | HBV | HBV | HBV | HCV & HBV | HCV | HCV | HCV | HCV-HIV | HCV-HIV | HCV-LT | HCV-LT |
| Proposed cutoff values (kPa) | 13.4 | 9.0 (N-ALT) | 11.0 | 14.0 | 14.6 | 12.5 | 14.8 | 11.8 | 14.6 | 12.0 | 12.5 |
| 12.0 (E-ALT) | |||||||||||
| Sensitivity (%) | 60 | 54 | 93 | 78 | 86 | 87 | 94 | 100 | 93 | 93 | 100 |
| Specificity (%) | 93 | 99 | 87 | 98 | 96 | 91 | 92 | 92.7 | 88 | 93 | 87 |
| NPV (%) | 88 | 67 | 99 | 82 | 97 | 95 | 98 | 82 | 94 | 99 | 100 |
| PPV (%) | 75 | 98 | 38 | 98 | 78 | 77 | 73 | 100 | 86 | 74 | 50 |
| Positive LR | 85 | 3.3 | 7.0 | 39.0 | 23.1 | 9.7 | 11.3 | 13.7 | 7.8 | 14.0 | 7.7 |
| Negative LR | 0.43 | 0.7 | 0.08 | 0.2 | 0.1 | 0.1 | 0.07 | 0 | 0.1 | 0.1 | 0 |
| AUROC | 0.93 | 0.88 | 0.93 | 0.96 | 0.97 | 0.95 | 0.99 | 0.97 | 0.95 | 0.90 | 0.98 |
ALT, alanine aminotransferase; AUROC, area under receiver operating characteristics curves; E-ALT, elevated ALT; HBV, hepatitis B virus infection, HCV, hepatitis C virus infection; HCV-HIV, hepatitis B virus and human immunodeficiency virus co-infection; HCV-LT, hepatitis C virus infection recurrence after liver transplantation; LR, likelihood ratio; N-ALT, normal ALT; NPV, negative predictive value; PPV, positive predictive value.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




