| Clin Mol Hepatol > Volume 20(3); 2014 > Article |
ABSTRACT
Background/Aims
This study assessed the antiviral efficacy and safety of tenofovir disoproxil fumarate (TDF) for up to 12 months in Korean treatment-naïve chronic hepatitis B (CHB) patients.
Methods
A total of 411 treatment-naïve CHB patients who had been treated with TDF for at least 3 months (median 5.6) were consecutively enrolled. Clinical, biochemical, virological parameters and treatment adherence were routinely assessed every 3 months.
Results
The median age was 51.3 years, 63.0% of the patients were male, 49.6% were HBeAg (+), and 210 patients had liver cirrhosis. The median baseline HBV DNA was 5.98 (SD 1.68) log10 IU/mL. Among the patients completing week 48, 83.3% had a complete virologic response (CVR, <12 IU/mL by HBV PCR assay), and 88.2% had normalized levels of alanine aminotransferase (ALT). The cumulative probabilities of CVR at 3, 6, 9 and 12 months were 22.8%, 53.1%, 69.3% and 85.0%. During the follow-up period, 9.8% patients achieved HBeAg loss and 7.8% patients achieved HBeAg seroconversion. There was no virological breakthrough after initiating TDF. The most common TDF-related adverse event was gastrointestinal upset, and three patients discontinued TDF therapy. However, no serious life-threatening side effect was noted.
Hepatitis B virus (HBV) infection is a leading cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma (HCC). It has affected over 400 million people worldwide.1 Untreated HBV infection may eventually lead to severe liver disease, including liver failure, cirrhosis and HCC, necessitating proper treatment.
In the past, lamivudine (LAM) was used extensively worldwide to treat HBV due to its relatively low cost (available generically in some countries) and favorable tolerability.2 However, several studies regarding high rate of HBV polymerase gene mutation and LAM drug resistance during drug administration were reported, ranging from 14-32% after 1 year, and 60-70% on 5 year treatment,2 which can cause serious clinical consequences.3,4,5 Thus, increasing evidence supports the use of alternative antiviral agents as the primary treatment option for CHB.6
Although preferred treatments may vary among countries and regions, and are dependent on the HBV genotypes that are prevalent in the region, present CHB treatment guidelines now recommend highly potent drugs, such as tenofovir disoproxil fumarate (TDF), along with entecavir as first-line therapeutic agents.7,8 Entecavir is an effective monotherapy in treatment-naïve patients that has a low resistance rate (1.2%), with up to 6 years of treatment.9,10 TDF has provided potent, long-term suppression of HBV replication for up to 5 years with no evidence of resistance based on international, multicenter phase III studies.11 However, these clinical trials focused mainly on genotype A and D. Data concerning CHB patients with genotype C, which is known to infect almost all HBV patients in Korea (>95%), are lacking.12 In addition, currently available data derived from these clinical trials often differ from those of real-world clinical practice including heterogeneous patients with other conditions. Therefore, "real-life" data are required to verify the validity of the findings of clinical studies and to continue monitoring of adverse events.
TDF, an analogue of adenosine 50-monophosphate, suppresses viral genome replication by inhibiting HBV DNA polymerase activity via competition with the natural substrate.13 It was approved for the treatment of CHB by the Korean Food and Drug Administration in December 2012. One year has passed since TDF became available on the market. This "real-life" study was performed to evaluate its efficacy and safety in treatment-naïve Korean CHB patients for up to 12 months.
We included consecutive na├»ve CHB patients who started TDF at 300 mg daily for at least 3 months in Severance Hospital, Seoul, Korea from December 2012 to December 2013. These patients were identified using the Severance Hospital Liver Disease Cohort Registry (SOLID CORE), which is an internal web-based electronic medical record that encompasses CHB patients treated with antiviral therapy at the Severance Hospital, Yonsei University College of Medicine. The inclusion criteria for this study were age Ōēź20 years, serum hepatitis B virus surface antigen present Ōēź 6 months, serum GFR 50 > mL/min/1.73 m2, more than 3 month treatment with TDF, and therapy na├»ve hepatitis B patient, defined as patients na├»ve to TDF who had no other antiviral therapy, such as interferon and other nucleosides, for at least 6 months prior to TDF therapy. Exclusion criteria were treatment with immunomodulatory drugs, current corticosteroid usage, coinfection with hepatitis C and/or D virus or HIV, and serious concurrent medical illness. All patients' laboratory tests at baseline and every 3 months from the starting point were analyzed. All patients were followed up regularly, and measurements were taken of HBV DNA, HBV serology, liver biochemistry and renal function, along with HCC surveillance and assessment of drug compliance. The patients' follow-up period ranged from 3 to 12 month (median 5.6). This study was approved by the local institutional review board and conducted in accordance with the principles set forth in the Declaration of Helsinki.
The primary end point of this study was complete virological response (CVR), which was defined as HBV DNA < 12 IU/mL (lower limit of detection) at any point during therapy. Secondary end points included biochemical response, mean decrease of HBV DNA titer, mean decrease in the Child-Pugh score in patients with decompensated liver cirrhosis, and HBeAg loss or seroconversion during the on-treatment follow up period. An on-treatment ALT flare was defined as an increase of 5x ALT Ōēź upper limit of normal (ULN; 46 IU/L) at any time during therapy. Liver cirrhosis was diagnosed based on clinical findings, ultrasonographic findings of a blunted, nodular liver edge accompanied by splenomegaly (>10 cm) with a low platelet count (< 100,000/┬ĄL), and/or by pathologic confirmation.
Microsoft Excel 2010 software (Microsoft, Seattle, WA, USA) was used for the database, and all statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL). Continuous variables were summarized as the median (range) or mean ┬▒ SD. Cumulative probabilities were estimated using Kaplan-Meier analysis and continuous variables were compared using the t-test. P-value < 0.05 was considered statistically significant.
A total of 1487 patients started TDF between December 2012 and December 2013 at the Severance Hospital, Yonsei University College of Medicine. Among them, 37 patients were treated with primary prophylactic TDF therapy and 9 had a serum GFR 50 < mL/min/1.73 m2. 761 patients were on antiviral therapy immediately before changing to TDF and 165 patients were treated with TDF and another antiviral agent. Another 104 patients had TDF treatment for less than 3 month or were lost during the follow-up period. As a result, a total of 411 patients with naïve CHB were enrolled (Table 1). The median age was 52 years (range 22-86). HBeAg positivity was noted in 204 (49.6%), cirrhosis was present in 210 (51.0%), and decompensated cirrhosis was in 64 (15.5%) patients. The baseline HBV DNA was 5.98 ± 1.68 log IU/mL.
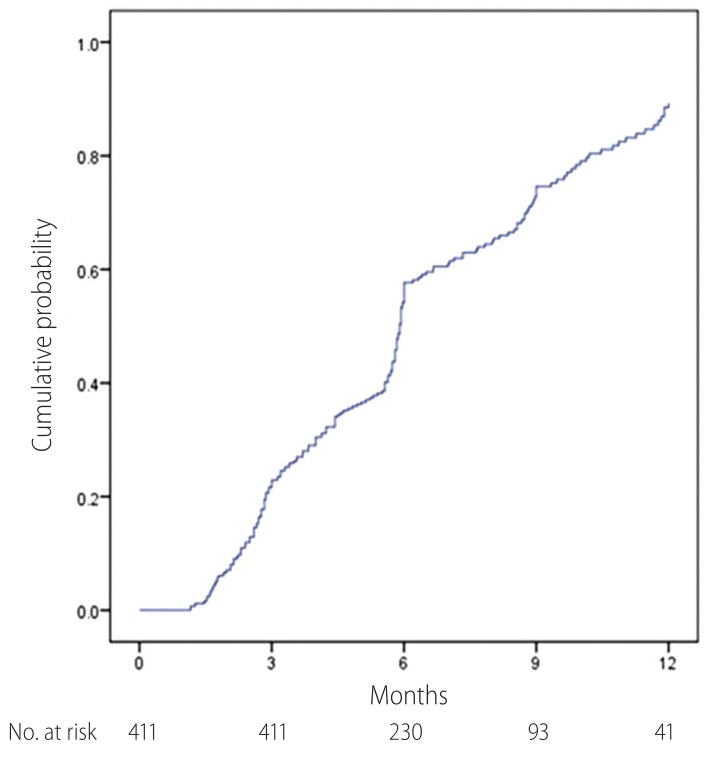
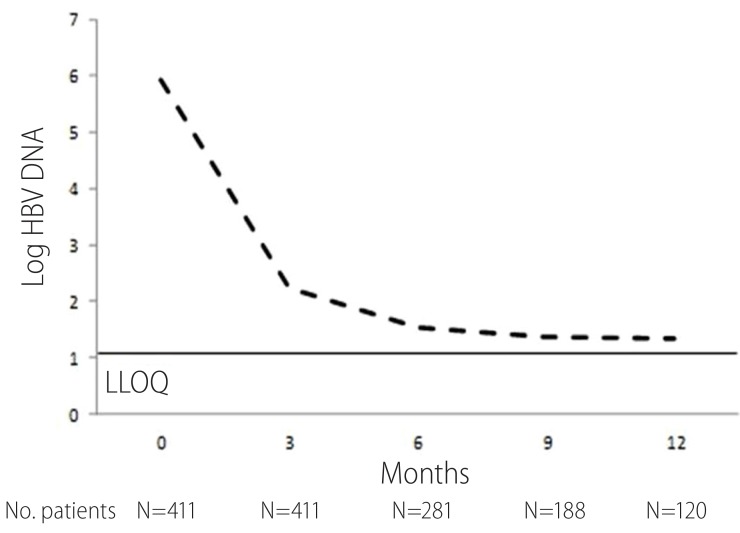
The proportion of patients with CVR is shown in Table 2. After 12-month treatment, 83.3% of total patients showed CVR. In the study population, virological response rate was quite similar and irrelevant to HBeAg and liver cirrhosis status. The cumulative probability of CVR is shown at Fig 1. The cumulative probabilities of CVR at 3, 6, 9 and 12 months were 22.8 %, 53.1%, 69.3% and 85.0%. At month 12, mean decline in serum HBV DNA level was -4.52 (log 5.92 in baseline and log 1.35 in month 12), which was statistically significant (95% CI 4.12-4.79; P<0.001) (Fig 2.). In HBeAg positive and negative patients, mean decrease of HBV DNA after 12 month was -5.19 and -3.94 respectively, while in patients with liver cirrhosis and without cirrhosis was -4.21 and -4.96. During 12 month follow-up period, no patient showed virological breakthrough or antiviral agent resistance.
The proportion of patients with biochemical response is shown in Table 3. After 12-month treatment, 88.2% of patients showed biochemical response. The biochemical response rate was similar when analyzed according to HBeAg and liver cirrhosis status (Table 3).
The proportion of patients achieving HBeAg loss was observed in 20 patients (9.8%), and seroconversion in 16 out of 204 (7.8%) patients, after median treatment duration of 6 months. Sixty three (15.3%) patients had decompensated liver cirrhosis, with a baseline mean Child-Pugh score in these patients were 7.03 (┬▒ 2.15 SD). Follow up mean score declined to a level of 6.95, 6.62, 6.12, and 6.06 at month 3, 6, 9 and 12.
The safety and tolerability profiles of TDF are shown in Table 4. Most of the adverse events were mild or moderate in severity and transient. Over 1 year, adverse events possibly related to TDF therapy were observed in 71 (17.2%) patients in the safety population. Common drug-related adverse events were on-treatment ALT flare in 17 (4.1%) patients, abdominal discomfort 15 (3.6%), nausea 11 (2.6%), poor oral intake, and fatigue. An increase in serum creatinine (Cr) of more than 0.2mg/dL was reported in 12 patients (2.9%). Drug discontinuation was reported in three patients (0.7%) due to gastrointestinal symptoms in all cases. There were two cases of gastrointestinal discomfort, one of which had general weakness and poor oral intake. Side effects occurred within 3 month after TDF therapy; these patients were not included in this study cohort, and all were switched to other antiviral agents. Twenty two patients were diagnosed HCC before treatment and one patient was diagnosed with HCC after 3 months. Three patients had undergone liver transplantation, and eight patients had died during the follow-up period.
Traditional treatment for chronic hepatitis B aims to normalize ALT, decrease HBV DNA titer, HBeAg clearance or seroconversion to HBeAg negativity, and improve liver histology. However, updates in the management of chronic hepatitis B increasingly emphasize a reduced HBV DNA titer as a better indication of treatment response, since it is associated with a decreased risk of development of liver cirrhosis or HCC as well as other liver-related events.14,15 An ideal drug for treating CHB has superior efficacy and lower risk of resistance and adverse effects. The current study was performed to analyze the antiviral effect, adverse effects, and outbreak of resistance after 48 week of TDF treatment.
In this "real-life" study, we have demonstrated that TDF as an initial therapy for naïve CHB patients was associated with favorable virological and biochemical response rates. This favorable treatment result was equally noted in the study population regardless of HBeAg serostatus and liver cirrhosis status, which is comparable to that of previous studies.16,17 The virological response rate seems to be lower in HBeAg positive patients, possibly due to the higher baseline HBV DNA titer. In addition, the mean decrease in HBV DNA titer in this study was relatively lower compared to other studies, which may have been attributable to the low baseline HBV DNA titer of the baseline study population.18 Rates of virological response, biochemical response, and the change in HBV DNA titer were equivalent even in patients with HCC.
Additionally, our data showed that TDF treatment in decompensated liver cirrhosis was associated with improvement in the Child-Pugh score over time.13,16 TDF is expected to improve liver function even in decompensated liver cirrhosis by suppressing HBV DNA replication, consistent with previous reports.19,20 However, some patients suffered deteriorated liver function, leading to liver transplantation or even fatality despite the antiviral therapy. This indicates that timely treatment may delay liver disease progression and development of HCC, avoiding the need for liver transplantation; however, when past a clinical threshold, high dose nucleoside analogue treatment is not always lifesaving in patients with decompensated liver cirrhosis.
No antiviral resistance was observed in our study, consistent with the results of previous studies, which constitutes a distinct advantage over other antiviral agents.21 However, the rate of HBeAg seroconversion/loss was relatively lower, perhaps due to the retrospective nature of the study which prohibited verification of HBeAg serostatus and anti-HBe status.13,16
TDF was generally well tolerated in this study, only with a few discontinuations due to adverse events and no life-threatening adverse events. The overall safety profile of TDF in this study was similar to that of previous reports,13,16 but due to the retrospective nature of this study, mild side effects may not have been recorded and not all adverse events, including elevated amylase, creatine kinase, glucose, and phosphate, were monitored regularly during the treatment period. As a result, most of the recorded adverse events were gastrointestinal and renal-related, meaning that the incidence of side effects was somewhat lower than in the literature.16 Three patients could not tolerate the therapy because of adverse gastrointestinal effects, and were switched to other nucleos(t)ide analogs.
One major strength of this study is that it was conducted in Korea, where CHB infection is endemic. Several previous studies have demonstrated the efficacy and safety of TDF;13,17,18,22,23 however, most of these were conducted in Western countries. Although the baseline genotype was not measured, the majority of CHB patients in Korea have genotype C,12 which has been associated with a poor clinical outcome and response to antiviral therapy.24,25 Clinical practice data have demonstrated the efficacy and safety of TDF in the treatment of CHB infection even in a highly prevalent area with an antiviral resistant strain.
This study had several limitations. First, its retrospective nature affected the collection of data and analysis of possible confounding factors. However, this study was a retrospective evaluation of a prospectively collected database, the Severance Hospital Liver Disease Cohort Registry (SOLID CORE). Therefore, the study included consecutive patients who received TDF and was intended to examine clinical outcomes in a "real-life" clinical setting.
In summary, this is the first study to provide 12-month data on the efficacy and safety of TDF in naïve CHB patients in Korea. TDF is a potent and safe drug for treatment of naïve CHB patients, but due to its higher cost compared to other antiviral agents, it may not be feasible to use for an extended period. Additional studies with larger sample sizes are required to evaluate the response rates, efficacy, incidence of various adverse events, and decrease in liver-related events following HBV DNA suppression.
Acknowledgments
We thank Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for assistance with the figures.
REFERENCES
1. Lee JM, Ahn SH, Kim HS, Park H, Chang HY, Kim do Y, et al. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology 2011;53:1486-1493. 21520167.


2. Marcellin P, Asselah T, Boyer N. Treatment of chronic hepatitis B. J Viral Hepat 2005;12:333-345. 15985003.


3. Yuen MF, Kato T, Mizokami M, Chan AO, Yuen JC, Yuan HJ, et al. Clinical outcome and virologic profiles of severe hepatitis B exacerbation due to YMDD mutations. J Hepatol 2003;39:850-855. 14568270.


4. Liaw YF. Management of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. J Gastroenterol Hepatol 2002;17(Suppl 3):S333-S337. 12472959.


5. Paik YH, Han KH, Hong SP, Lee HW, Lee KS, Kim SO, et al. The clinical impact of early detection of the YMDD mutant on the outcomes of long-term lamivudine therapy in patients with chronic hepatitis B. Antivir Ther 2006;11:447-455. 16856618.



6. Park MS, Kim BK, Kim KS, Kim JK, Kim SU, Park JY, et al. Antiviral efficacies of currently available rescue therapies for multidrug-resistant chronic hepatitis B. Clin Mol Hepatol 2013;19:29-35. 23593607.



7. Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531-540. 22114089.



8. KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol 2012;18:109-162. 22893865.



9. Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology 2009;49:1503-1514. 19280622.


10. Chon YE, Kim SU, Lee CK, Heo J, Kim JK, Yoon KT, et al. Partial virological response to entecavir in treatment-naive patients with chronic hepatitis B. Antivir Ther 2011;16:469-477. 21685534.


11. Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 2011;140:132-143. 20955704.


12. Song BC, Cui XJ, Kim H. Hepatitis B virus genotypes in Korea: an endemic area of hepatitis B virus infection. Intervirology 2005;48:133-137. 15812186.


13. Duarte-Rojo A, Heathcote EJ. Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B. Therap Adv Gastroenterol 2010;3:107-119.



14. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73. 16391218.


15. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678-686. 16530509.


16. Buti M, Homs M. Tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Expert Rev Gastroenterol Hepatol 2012;6:413-421. 22928893.


17. Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in 'real-life' settings: from clinical trials to clinical practice. J Viral Hepat 2012;19:377-386. 22571899.



18. Ceylan B, Yardimci C, Fincanci M, Eren G, Tozalgan U, Muderrisoglu C, et al. Comparison of tenofovir and entecavir in patients with chronic HBV infection. Eur Rev Med Pharmacol Sci 2013;17:2467-2473. 24089225.

19. Ahn SH, Chan HL, Chen PJ, Cheng J, Goenka MK, Hou J, et al. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int 2010;4:386-395. 20305758.



20. Miquel M, Nunez O, Trapero-Marugan M, Diaz-Sanchez A, Jimenez M, Arenas J, et al. Efficacy and safety of entecavir and/or tenofovir in hepatitis B compensated and decompensated cirrhotic patients in clinical practice. Ann Hepatol 2013;12:205-212. 23396731.


22. van Bommel F, Wunsche T, Schurmann D, Berg T. Tenofovir treatment in patients with lamivudine-resistant hepatitis B mutants strongly affects viral replication. Hepatology 2002;36:507-508. 12143063.


23. Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, Dinh P, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology 2013;58:505-513. 23364953.



Table┬Ā1.
Baseline characteristics of the subjects
Table┬Ā2.
Proportion of patients with virological responses (undetectable HBV DNAa) according to HBeAg and liver cirrhosis status
Table┬Ā3.
Proportion of patients with biochemical responses (ALT normalizationa) according to HBeAg and liver cirrhosis status
Table┬Ā4.
Incidence of adverse events in the TDF treated population



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




