Long-term outcomes of two rescue therapies in lamivudine-refractory patients with chronic hepatitis B: combined lamivudine and adefovir, and 1-mg entecavir
Article information
Abstract
Background/Aims
Adefovir (ADV) and lamivudine (LAM) combination therapy (ADV+LAM) has been a useful option for patients with LAM-resistant (LAM-r) chronic hepatitis B (CHB). However, the long-term outcomes of LAM+ADV and 1-mg entecavir (ETV) rescue therapies have still been limited. The aim of this study was to determine the long-term outcomes of these two rescue therapies.
Methods
Sixty patients with LAM-r CHB underwent rescue therapy with LAM+ADV (n=36) or 1-mg ETV (n=24). We determined the duration of rescue therapy, timing and type of mutation, undetectable serum hepatitis B virus (HBV) DNA by PCR (lower limitation of detection, < 140 copies/mL), biochemical response (alanine aminotransferase < 40 IU/mL), and the incidence of hepatitis B virus e antigen (HBeAg) seroconversion and virologic breakthrough.
Results
Baseline characteristics did not differ between the two therapy groups. The duration of rescue therapy was 56 months (range, 14-100 months) in the ADV+LAM group and 42 months (range, 12-73 months) in the ETV group (P=0.036). The cumulative rates of HBV DNA undetectability and HBeAg seroconversion up to 6 years were 88.6% and 43.0%, respectively, in the ADV+LAM group, and 45.8% and 31.8% in the ETV group. The rate of virologic breakthrough and resistance was 14.4% in the ADV+LAM group and 71.9% in the ETV group (P=0.001).
Conclusions
Combination of LAM and ADV therapy for up to 6 years achieved modest rates of virological suppression and resistance. ETV is not an optimal therapy because the risk of viral breakthrough to ETV increases over time.
INTRODUCTION
Hepatitis B virus (HBV) infection poses a major global health concern based on the approximately 350 million individuals suffering from chronic hepatitis B (CHB) worldwide. Studies have demonstrated that the risk of liver diseases progression in patients with CHB is associated with elevated HBV-DNA levels.1,2 Therefore, the ideal goal of antiviral therapy is to permanently clear HBV infection, possibly before irreversible damage is established by suppressing long-term HBV-DNA replication.3
LAM, ADV, and ETV are nucleoside or nucleotide analogs that have been approved for treating CHB. These drugs have inhibitory effects on HBV polymerase/reverse transcriptase activity, but no direct effects on covalently closed circular DNA (cccDNA) synthesis; thus, prolonged therapy is usually required to ensure maintained or sustained HBV suppression.4
In the early period, LAM was the first drug to be approved for treatment of CHB.4,5 Worldwide studies of LAM in patients with CHB have demonstrated good efficacy based on improved hepatic histology, Hepatitis B virus e antigen (HBeAg) seroconversion.6,7 However, long term use of LAM leads to emergence of a resistant HBV (YMDD) mutant. LAM resistance increases progressively during treatment at rates of 15-20% annually, exceeding 67-70% after 5 years of treatment.8,9,10
Therefore, LAM monotherapy is not currently considered as a first-line treatment. Previous rescue therapy for the treatment of LAM-resistant chronic hepatitis B (LAM-r CHB) infected patients included switching to entecavir (ETV) and adding adefovir (ADV) or tenofovir (TDF).4 As a rescue therapy, ETV monotherapy resulted in continued viral suppression and biochemical and serologic responses; however, sequential ETV therapy resulted in a 5-year cumulative probability of genotypic ETV resistance of 51%.11 In addition, in several recent studies, LAM plus ADV combination therapy was effective and produced longer-lasting effects than switching to ETV monotherapy in treating LAM-r CHB patients.12,13 The 2009 American Association for the Study of Liver disease (AASLD) Guidelines indicated that ETV was not the optimal treatment option and recommended adding ADV to treat LAM-r CHB patients if TDF was not available.4 Up to now, there are some data on the long-term efficacy of two rescue therapies in patients with LAM-r CHB. The aim of this retrospective study was to clarify the long-term efficacy of the two rescue therapies in patients with LAM-r CHB.
MATERIALS AND METHODS
Study population
Data reports were collected retrospectively from outpatient visit charts from the hospital clinic. A total of 60 consecutive LAM-r CHB patients (43 male, mean age 52 years) were enrolled in this study from the Chung-Ang university hospital, Seoul, Korea, between January 2007 and October 2008. All patients had been originally diagnosed with compensated CHB and treated with LAM. They all developed viral breakthrough after mean 21 months with LAM treatment. The median duration of rescue treatment was 51 months (range: 12-100 months). They were put on combination therapy with LAM 100 mg/day plus ADV 10 mg/day or ETV 1.0 mg/day rescue therapy.
Study measurements
All patients were followed by means of clinical, biochemical, serological, and virologic evaluation every year. Routine liver biochemical tests including aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, bilirubin and prothrombin time were performed using a sequential multiple auto analyzer. HBeAg and antibody to HBeAg (anti-HBe) were tested with enzyme-linked immunoassay (Modular E-170 Automatic Immunochemistry system, Roche). Serum HBV DNA concentration was determined by a commercially available quantitative assay (Light cycler 2.0, Roche). The sensitivity of the assay is approximately 116 copies/mL. Genotyping and analyzing of resistance of LAM, ADV, or ETV with restriction fragment mass polymorphism (RFMP) analysis were performed when viral breakthrough was detected. Confirmation of genotypic LAM resistance was seen in all 60 patients.
Definitions
Virologic response was defined as undetectable serum HBV-DNA by PCR. The biochemical response was defined as the decline of serum ALT levels to the normal range. A virological breakthrough was defined as > 1log10 increase in serum HBV-DNA level above nadir.
Study end point
The primary end point was the comparison of proportions of CHB patients with undetectable serum HBV-DNA between the ADV plus LAM group and ETV group every year during six years of treatment. The secondary end point included the proportions of CHB patients with HBeAg seroconversion, virological breakthrough, and the genotypic resistance of ADV and ETV. In patients with drug modification, the data were included until cross-over and classified as treatment failure.
Statistical analysis
All data were analyzed using the statistical package SPSS (version 18). Patient characteristics were compared by use of the chi-squared test or Fisher's exact test for categorical variables and Student's t-test for continuous variables. Serum HBV-DNA levels were logarithmically transformed for analysis. Cumulative probabilities of undetectable HBV-DNA, HBeAg seroconveresion, and virologic breakthrough were analyzed by Kaplan-Meier method and Log rank test. P<0.05 was considered statistically significant.
RESULTS
Study population
Of the 60 patients enrolled, 36 patients received rescue therapy with LAM plus ADV, and 24 patients received ETV 1 mg rescue therapy daily. Baseline demographical, clinical and laboratory characteristics are given in Table 1. In this study, 'baseline' refers to the time point at which the LAM resistance occurred and rescue therapy was started. At baseline there was no significant difference in gender, age, serum ALT and HBV-DNA level, HBeAg status, liver cirrhosis and duration of LAM therapy. However, the duration of rescue therapy in ADV combination and ETV group was 56 months (range 14-100 months) vs 42 months (range 12-73 months) (P=0.014). The rates of sustained rescue treatment was 77.8% (28/36) vs 45.8% (11/24) (P=0.011). In ADV combination group, 8 patients was discontinued the combination therapy. Five of these were switched to ETV plus ADV, and three were switched to ADV monotherapy. In the ETV group, 13 patients were discontinued the rescue therapy. Seven of these were switched to ETV plus ADV, five were switched to LAM plus ADV, and one was switched to telbivudine plus ADV.
Virologic response
The cumulative rates of becoming HBV-DNA undetectable in the ADV combination group were higher than in ETV group. In ADV combination group, the cumulative rates were 22.2%, 26.3%, 36.5%, 65.8%, 88.6% and 88.6%, annually. And, in the ETV rescue group, the cumulative rates each year were 21.8%, 29.2%, 45.8%, 45.8%, 45.8% and 45.8% (Fig. 1). During the follow-up period, the cumulative rate of HBV-DNA undetectability was significantly higher in ADV combination group (P=0.016).
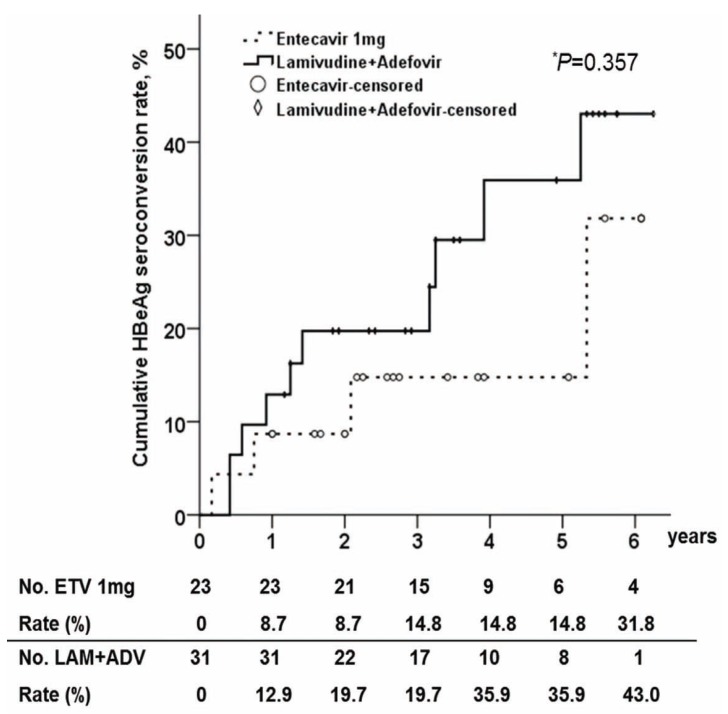
Serological response
Among HBeAg positive patients at baseline, the proportion of patients who achieved HBeAg seroconversion was slightly higher in the ADV combination group compared with in ETV group (Fig. 2). After 6 years of treatment, the cumulative rate of HBeAg seroconversion in the ADV combination group was 43.0%, and the rate in ETV group was 31.8%. However, there was no statistical difference in the cumulative rates of HBeAg seroconversion between two groups (P=0.375).
Biochemical response
In ADV combination group, the proportions of subjects whose ALT levels had normalized annually were 80%, 82.4%, 79.4%, 78.1%, 79.5% and 84%. In ETV rescue group, the proportions were 66.7%, 81.8%, 73.7%, 80%, 84.5% and 91.7%. No significant differences were found between two groups in biochemical response.
Virologic breakthrough and genotypic resistance
The cumulative virologic breakthrough rate was significantly lower in the ADV combination than ETV rescue group (P=0.001). The cumulative virologic breakthrough rates at 1, 2, 3, 4, 5 and 6 years in each group were 0%, 9.3%, 11.1%, 14.4%, 14.4% and 14.4%, vs 4.2%, 21.6%, 40.0%, 55.6%, 61.1% and 71.9%, respectively (Fig. 3).
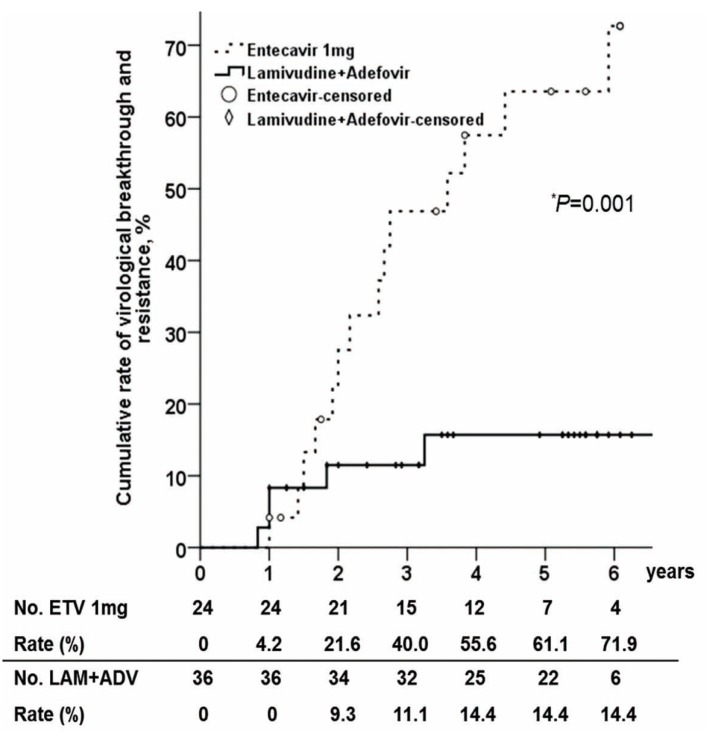
Cumulative rates of virologic breakthrough and resistance in patients treated with lamivudine plus adefovir therapy and entecavir monotherapy. *P-value from Log rank test. ETV, entecavir; LAM+ADV, lamivudine plus adefovir.
Analysis of genotypic resistance was performed when viral breakthrough was detected. In ADV combination group, virologic break though showed in 10 patients. Among them, five (50%) of those patients had genotypic resistance and five patients had low viremia (184-9,106 copies/mL) without genotypic resistance. The median period from the rescue therapy to genotypic resistance was 26 months (13-39 months). In this group, mutations of A181T/V/S, and N236T were found (Table 2).
In ETV rescue group, virologic breakthough showed in 13 patients, and all patient shad genotypic resistance. The median duration from the rescue therapy to genotypic resistance was 21 months (10-33 months). The LAM and ETV-resistance mutational patterns are given in Table 3.
DISCUSSION
Preventing HBV antiviral drug resistance to nucleoside/nucleotide analogues and appropriate management when resistance occurs have become a major focus in the management of CHB. The 2009 AASLD Guidelines indicated that ETV was not the optimal treatment option and recommended adding ADV to treat LAM-r CHB patients if TDF was not available.4,12,13,14,15
Our findings showed that the virologic response was less effective for patients who switched to ETV than for patients receiving ADV combination therapy as rescue treatment for LAM-r CHB patients who require long-term antiviral therapy. These results were in agreement with those in recent reports which showed a trend toward an improved virologic response in the add-on ADV group than in the ETV group.13,14,15 The explanation of the different virologic response between the two groups was the presence of HBV strains with limited sensitivity to ETV.
LAM resistance mutations have previously been shown to result in reduced susceptibility to ETV in vitro,16 and ETV had positive selective pressure on LAM-resistant mutants in vivo.17 ETV resistance leading to virologic breakthrough emerges more frequently in the presence of the LAM-resistant mutations because rtM204V and rtL180 M are necessary in combination with an additional substitution at rtS202, rtT184, or rtM250 to restore the replication competence and fitness of HBV variants resistant to ETV.11,17,18 This cross-resistance of ETV is a possible explanation for the lower efficacy of ETV-switching therapy compared with ADV add-on LAM therapy in patients with prior LAM resistance. It might be important to recognize preexisting resistance in order to decide on the appropriate antiviral therapy and avoid multi-drug resistance that results from sequential therapy.19,20
Adefovir has both merits and demerits, especially in patients with LAM resistance. Several previous studies have shown that ADV-resistance mutations are infrequent in combination therapy with LAM.21,22,23 In addition, ADV has a cost benefit in its long-term experience and safety. However, limited efficacy was seen in patients with a higher baseline HBV-DNA level. In addition, add-on ADV should be implemented early because earlier addition of ADV at the time of virologic breakthrough (when the HBV-DNA levels are low and before the development of biochemical breakthroughs) is associated with a significantly better long term outcome in terms of HBV-DNA suppression, and development of ADV-resistant HBV.24 As the baseline HBV-DNA level was an independent predictor of the virologic response, the early addition of ADV for preventing an increase in HBV-DNA and individualized strategy based on the baseline HBV-DNAlevel is need to be considered.15,22
One previous study in LAM-resistant patients demonstrated that ADV plus LAM combination therapy did not result in the development of resistance to ADV over a period of 3 years. Other studies reported an emergence rate of ADV resistance during ADV plus LAM combination therapy of 1% at 1 year and 4% at 3 years, however, no virological rebound was noted. In our study, the median period from the rescue therapy to genotypic resistance was 26 months (13-39 months), and the mutations of A181V/T/S and N236T were found in ADV combination group.
Recently, TDF has become an effective alternative for the treatment of patients with LAM- or ADV-resistant CHB.25,26,27 Studies of TDF monotherapy and TDF plus ETV are ongoing in patients with suboptimal responses to other nucleotide/nucleoside analogs. A combination of high potencydrugs such as TDF plus ETV therapy may be more beneficial in patients with a higher baseline HBV DNA, because it is more potent and has a genetic barrier, although this remains to be clarified in the future.
This retrospective study has some limitations. Firstly, our study was not randomized and our results are prone to selection bias. The similarity, however, of the baseline characteristics of the patients between the two groups may overcome the weak points of this comparison. Secondly, patients with genotypic resistance and breakthrough were assigned cross-over rescue therapy. Therefore, we could not see the exact long term outcome of two treatment arms especially in the entecavir 1 mg monotherapy group. Lastly, the numbers of patients are small in each groups, the validity of the results is likely to be clinically decreasing.
In conclusion, LAM-resistant variants of HBV have a reduced resistance barrier to ETV and patients with LAM resistance are likely to benefit from combination therapy without cross-resistance, because the risk of developing ETV resistance is relatively high in such patients. Therefore, LAM plus ADV combination therapy was more effective and longer lasting than switching to ETV monotherapy in treating LAM-r CHB patients who require long-termantiviral therapy. Add-on ADV plus LAM therapy is still preferred as rescue treatment for LAM-r CHB patients.
Notes
The authors have no conflicts to disclose.
Abbreviations
ADV
adefovir
ALT
alanine aminotransferase
CHB
chronic hepatitis B
ETV
entecavir
HBeAg
hepatitis B virus e antigen
HBV
hepatitis B virus
LAM
lamivudine
LAM-r CHB
lamivudine-resistant chronic hepatitis B
TDF
tenofovir