INTRODUCTION
Hepatitis B virus (HBV) is the most common cause of chronic liver disease including liver cirrhosis and hepatocellular carcinoma.1 Recently, potent oral nucleos(t)ide analogues, including entecavir and tenofovir, have been widely used in the treatment of chronic hepatitis B (CHB).2,3,4,5 The potent antiviral treatment in patients with CHB can suppress the viral replication and prevent progression to cirrhosis, hepatic failure and HCC.6,7,8,9,10,11,12
During the natural course of CHB infection, spontaneous HBeAg seroconversion, defined as loss of HBeAg and detection of anti-HBe in a person who was HBeAg positive and anti-HBe negative,4 occurred frequently in the immune reactive phase in patients with HBeAg-positive CHB ranging from 2-17% per year depending on serum alanime aminotranferase (ALT) levels.13,14,15,16,17,18,19,20,21 Spontaneous HBeAg seroconversion is usually followed by normalization of serum aminotransferase levels and sustained suppression of HBV DNA (<2,000 IU/ml) and is associated with favorable long-term outcomes in patients with CHB.13,22,23 Therefore, in HBeAg-positive CHB patients with elevated serum ALT, most guidelines recommend observation for 3-6 months before antiviral therapy to anticipate spontaneous HBeAg seroconversion.2,3,4,5
However, spontaneous HBeAg seroconversion occurred infrequently in patients with genotype C compared to those with other genotypes.15,16,24 Therefore, the strategy for initiation of antiviral therapy would be different according to the distribution of HBV genotype. The purpose of this study was to investigate the necessity of the waiting strategy before antiviral therapy in HBeAg-positive CHB patients in endemic areas of HBV genotype C infection.
PATIENTS AND METHODS
We prospectively enrolled HBeAg-positive CHB patients with HBV DNA levels of more than 20,000 IU/mL and ALT levels of more than two times the upper limit of normal (ULN) between June 2009 and September 2013 in Jeju National University Hospital. Patients were excluded if they had concurrent hepatitis C or other liver disease (alcoholism, autoimmune liver disease, toxic hepatitis, liver cirrhosis and hepatocelluar carcinoma). Pregnant women and patients who refused this study were also excluded.
The patients were followed without antiviral treatment to anticipate spontaneous HBeAg seroconversion during 6 months. They were followed at 1-3 month intervals or more frequently if clinically needed. At every visit, serial liver function tests, including ALT and bilirubin, and HBV DNA were measured. HBeAg and anti-HBe were analyzed by third-generation microparticle enzyme immunoassays using commercial enzyme immunoassay kits (Abbott, North Chicago, IL, USA). Serum HBV DNA levels were measured by the Cobas Amplicor HBV Monitor (detection limit, 60 IU/mL) (Roche Molecular Diagnostics, Pleasanton, CA, USA) before December 2009 or the Cobas AmpliPrep/Cobas TaqMan® HBV Test, v2.0 (detection limit, 20 IU/mL) (Roche Molecular Diagnostics) after DeDecember 2009.
Antiviral therapy was initiated promptly at any time if there was any evidence of biochemical (acute exacerbation of hepatitis B or aggravation of jaundice, defined by bilirubin ≥2 mg/dL) or symptomatic deterioration which disturbed the patients' daily life, and the patients wanted to receive antiviral therapy. Acute exacerbation of hepatitis B was defined as elevations of serum ALT levels to more than 10 times the ULN and more than twice the baseline value.4 In patients with biochemical deterioration, other causes of acute hepatitis were excluded.
Data analysis was performed using SPSS version 12.0 for windows (SPSS Corp, Chicago, IL, USA). The data were analyzed by the Student's t-test for continuous variables and the chi-square test for categorizing variables. A logistic regression analysis was used to identify the independent predictive factors for biochemical or symptomatic deterioration. P values less than 0.05 were considered significant.
The study protocol was approved by the ethics committees of our institution and written informed consents were obtained from the patients.
RESULTS
Patient characteristics
During the study period, 119 consecutive patients fulfilled the inclusion criteria. Among them, 29 patients were excluded because of exclusion criteria (n=19) and were lost to follow-up after the first visit (n=10). Therefore, 90 patients with HBeAg-positive CHB were analyzed. Baseline characteristics of the 90 patients are shown in Table 1. The mean age of the patients was 37.9 years and 60 patients (67.0%) were male. Fifty-four patients (60%) were vertically infected.
During follow-up, only one (1.1%) of 90 HBeAg-positive CHB patients achieved spontaneous HBeAg seroconversion at 2 months. In another patient, HBeAg loss, but not anti-HBe positive occurred at 3 months. However, in this patient, persistent elevation of HBV DNA above 2,000 IU/mL and elevation of ALT levels was observed over 6 months.
Biochemical deterioration was observed in 17 patients (18.9%; acute exacerbations in 8, aggravation of jaundice in 2, and both in 7 patients) and symptomatic deteriorations without biochemical deterioration occurred in 5 patients before 6 months.
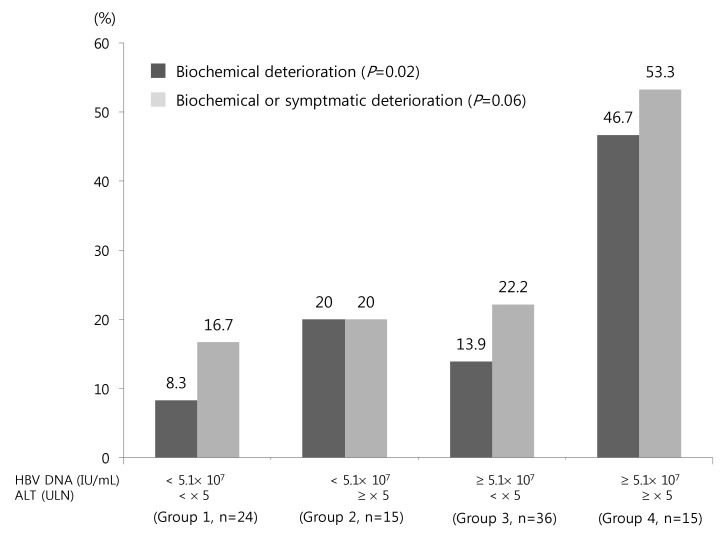
Biochemical deterioration occurred more often in patients with high ALT, high bilirubin and high HBV DNA levels in univariate analysis (Table 1). Among these, high serum ALT and HBV DNA levels were two independent risk factors for biochemical deterioration (Table 2). Serum HBV DNA levels were dichotomized at the levels of 5.1×107 IU/mL (median value). We categorized the patients into the following 4 groups: Group 1 (n=24), low HBV DNA (<5.1×107 IU/mL) and low ALT (< ×5 ULN); group 2 (n=15), low HBV DNA and high ALT (≥×5 ULN); group 3 (n=36); group 4 (n=15), high HBV DNA (≥5.1×107 IU/mL) and high ALT. The percentages of biochemical or symptomatic deteriorations in each group are demonstrated in Fig. 1.
In 17 patients with biochemical deterioration and 5 patients with symptomatic deterioration (n=22), immediate antiviral therapy was recommended before 6 months of follow up. Twenty patients received antiviral therapy (entecavir or tenofovir) promptly (mean 3 months, range 1-5 months). However, in 2 patients with acute exacerbation of hepatitis B during follow-up, antiviral therapy was initiated at 8 and 9 months as the patients wanted to delay the treatment for several months anticipating spontaneous HBeAg seroconversion. Among 17 patients with biochemical deterioration during follow-up, a patient received living-related liver transplantation because of acute-on-chronic liver failure25 even introducing entecavir therapy (Fig. 2).
In remaining 68 patients without biochemical or symptomatic deterioration within 6 months, 56 patients received antiviral therapy at or after 6 months of follow-up (mean 12.9 months, range 6-48 months), 6 patients did not satisfy the treatment indication until the last follow-up, and 6 patients were lost to follow-up after 6 months. As a total, 78 of 84 patients (93%) who were followed, received antiviral therapy.
Antiviral therapy was initiated in 17, 14, 31 and 16 patients during follow-up in group 1, 2, 3 and 4, respectively. In each group, antiviral therapy was initiated at a mean of 8.9 months (3-32 months), 18.2 months (1-48 months), 9.8 months (2-36 months) and 6.1 months (1-18 months) in group 1, 2, 3 and 4, respectively.
DISCUSSION
The current study showed two important findings. First, the possibility of HBeAg seroconversion within 6 months is negligible in the endemic areas of HBV genotype C infection even in patients with elevated serum ALT levels. Second, biochemical deterioration occurred frequently during short-term follow-up, especially in patients with high ALT and high HBV DNA levels.
Previous studies showed that spontaneous HBeAg seroconversion occurred more often in patients with old age, high ALT, non-genotype C and low HBV DNA.15,16,17,18,19,26 In a Hong Kong study,17 HBeAg seroconversion rate at 5 years was 72.4% of the patients with ALT levels more than 2 times the ULN on clinical presentation. In addition, the possibility of HBeAg seroconversion within 3 months in patients with peak ALT levels 1.5-2 times the ULN, >2-5 times the ULN, and > 5 times the ULN were 27.2%, 35.6%, and 46.4%, respectively.17 In a Korean study, HBeAg seroconversion occurred in 35.5 % within 6 months in patients with ALT levels more than 5 times the ULN.18 Therefore, current guidelines for the treatment of CHB recommend observation for 3-6 months to anticipate spontaneous HBeAg seroconversion in HBeAg-positive CHB patients with HBV DNA> 20,000 IU/mL, and serum ALT more than 2 times the ULN.2,3,4,5
The geographic distribution of HBV genotypes are different.27 Almost all Korean patients with CHB are infected with HBV genotype C.18,28,29 Spontaneous HBeAg seroconversion occurred infrequently in patients with genotype C compared to those with other genotypes.15,16,24 A previous study showed that the cumulative rates of spontaneous HBeAg seroconversion at 1, 3, and 5 years were 6%, 24%, and 46% in genotype B patients and 0%, 18%, and 34% in genotype C patients, respectivly.15 It has been also reported that spontaneous HBeAg seroconversion occurred one decade later in genotype C patients compared to genotype B patients.15,16,24 It did not occur within 6 months, even in patients with ALT levels more than 4-5 times the ULN15,19 in patients with genotype C. In addition, the difference in the rate of HBeAg seroconversion was predominantly noted in patients who had elevated ALT levels on presentation. In patients with genotype B, HBeAg seroconversion was dependent on serum ALT levels on presentation. However, in patients with HBV genotype C, HBeAg seroconversion was not dependent on serum ALT levels.
In general, high serum ALT levels are considered a surrogate marker for vigorous host immune response caused by cytotoxic T-cell-mediated immune hepatolysis and eventual immune clearance of HBV.30,31 However, HBeAg seroconversion occurred only in 1.1% within 6 months of follow-up in this study. Previous studies15,19 and this data suggests that some abortive immune clearance mechanism might exist in patients infected with HBV genotype C.
A study in Korea reported that HBeAg seroconversion occurred in 35.5 % within 6 months in patients with ALT levels more than 5 times the ULN.18 We could not explain the exact mechanism of the discrepancy between the previous studies15,18,19 and our data. Previous study18 showed that non-vertical infection of HBV was one of the independent predictive factors for HBeAg seroconversion. In their study, 31% of the subjects were vertically infected.18 In our study, 60% of the subjects were vertically infected. The difference of the proportion of vertical infection in subject might partially explain the difference in HBeAg seroconversion rate within 6 months. Yoon et. al also reported that HBeAg seroconversion within 6 months in patients with elevated ALT (mean 179 IU/mL) levels was less than 3%.32
Acute exacerbation of hepatitis B may lead to hepatic decompensation and death from liver failure.20,33,34,35,36 In this study, acute exacerbation of hepatitis B or aggravation of jaundice developed in 17 patients (18.9%) and one patient received liver transplantation even after introducing antiviral because of acute-on-chronic liver failure following acute excerbation of hepatitis B. Jeng et al. reported that hepatic decompensation occurred in 5.1%, especially in patients with serum HBV DNA>1.55×109 copies/mL (around 3×108 IU/mL) in CHB patients with abrupt increase in ALT more than 5 times the ULN.36 In this study, in patints with high ALT ( ≥×5 ULN) and high viral load (HBV DNA ≥ 5.1×107 IU/mL), acute exacerbation of hepatitis B or aggravation of jaundice developed in 46.7% of the patients. Lok et al. also reported that acute exacerbationof hepatitis B occurred frequently in patient with ALT levels more than 5 times the ULN.35 In addtion, Kim et al. resported that patients with serum HBV DNA levels more than 2×106 IU/mL had liitle possibility of spontaneous HBeAg seroconversion.18 After spontaneous HBeAg seroconversion, those with genotype C were more likely to revert to the HBeAg-positive state24 and reactivation of hepatitis B18,37 occurred in 50-80% of patients during long-term follow-up.18,37 In addition, reactivation of hepatitis B occurred frequently in patients with genotype C compared with those with genotype C and serum ALT levels more than 5 times the ULN.37
Considering previous results18,35,36,37 and the results of our study, serum ALT and HBV DNA cut-off levels for immediate antiviral therapy might be 5 times the ULN and 5.1×107 IU/mL, respectively.
In conclusion, spontaneous HBeAg seroconversion in patients with HBeAg-positive CHB in the endemic area of genotype C infection was rare within 6 months. Biochemical deterioration was common. Therefore, immediate antiviral therapy should be considered, especially in patients with high ALT and HBV DNA levels.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




