Effects of candesartan and propranolol combination therapy versus propranolol monotherapy in reducing portal hypertension
Article information
Abstract
Background/Aims
Angiotensin receptor blockers (ARBs) inhibit activated hepatic stellate cell contraction and are thought to reduce the dynamic portion of intrahepatic resistance. This study compared the effects of combined treatment using the ARB candesartan and propranolol versus propranolol monotherapy on portal pressure in patients with cirrhosis in a prospective, randomized controlled trial.
Methods
Between January 2008 and July 2009, 53 cirrhotic patients with clinically significant portal hypertension were randomized to receive either candesartan and propranolol combination therapy (26 patients) or propranolol monotherapy (27 patients). Before and 3 months after the administration of the planned medication, the hepatic venous pressure gradient (HVPG) was assessed in both groups. The dose of propranolol was subsequently increased from 20 mg bid until the target heart rate was reached, and the candesartan dose was fixed at 8 mg qd. The primary endpoint was the HVPG response rate; patients with an HVPG reduction of >20% of the baseline value or to <12 mmHg were defined as responders.
Results
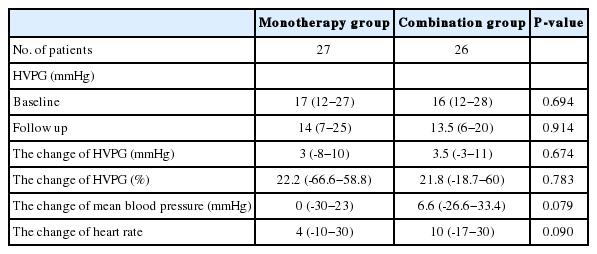
The mean portal pressure declined significantly in both groups, from 16 mmHg (range, 12-28 mmHg) to 13.5 mmHg (range, 6-20 mmHg) in the combination group (P<0.05), and from 17 mmHg (range, 12-27 mmHg) to 14 mmHg (range, 7-25 mmHg) in the propranolol monotherapy group (P<0.05). However, the medication-induced pressure reduction did not differ significantly between the two groups [3.5 mmHg (range, -3-11 mmHg) vs. 3 mmHg (range, -8-10 mmHg), P=0.674]. The response rate (55.6% vs. 61.5%, P=0.435) and the reductions in mean blood pressure or heart rate also did not differ significantly between the combination and monotherapy groups.
Conclusions
The addition of candesartan (an ARB) to propranolol confers no benefit relative to classical propranolol monotherapy for the treatment of portal hypertension, and is thus not recommended.
INTRODUCTION
Portal hypertension (PH) is a critical prognostic factor for patients with cirrhosis.1,2,3 The risk of PH related complications increases when hepatic venous pressure gradient (HVPG) elevates to more than 10 mmHg.4,5 Variceal bleeding is one of the most common and severe complications in patients with cirrhosis. The mortality rate of acute variceal bleeding reaches about 25%.6,7 An increase of portal pressure depends on elevated intrahepatic resistance (IHR) and amount of portal blood flow into the liver. Non-selective beta blocker (NSBB) decreases the latter one through reduction of cardiac output and splanchnic vasoconstriction and consequently reduces PH. Although NSBB is still the most essential medical approach in the management of PH until now, the response rate is just about 50-60% and only reduction of portal blood flow cannot satisfy clinical demands,8 and the needs for development of new medical approach especially to decrease IHR have increased.
The renin-angiotensin-aldosterone (RAA) system plays multiple key roles in the pathogenesis of chronic liver disease and portal hypertension. Angiotensin II which act on angiotensin II type 1 receptor (AT1-R) is a main actor in RAA system and elevated in liver cirrhosis.9,10 Increased angiotensinII accelerates hepatic fibrosis by activation of hepatic stellated cells (HSCs) and producing of extracellular matrix. In addition, the activated HSC by angiotensin II contracts and induce the increase of IHR.11,12 For this reason, The regulation of Angiotensin II using AT1-R blockers (ARB) has been expected as a new medical approach in the management of portal hypertension by decrease of IHR.13 However, AT1-R blockers alone did not show better effect than NSBB through many previous studies.11,14,15,16 Therefore, this study aimed to identify the combination effect of ARB and NSBB comparing with NSBB monotherapy in reducing of PH.
PATIENTS AND METHODS
Study population
Patients between 19 and 75 years of age with liver cirrhosis who visited the Wonju College of Medicine Wonju Severance Christian Hospital from January 2009 to July 2012 were considered eligible for the study. All cirrhotic patients who admitted hospital to get an intensive examination for PHT or to manage the already developed PHT related complications such as variceal hemorrhage or ascites were consider for this study. The target inclusion criteria were any etiology originated cirrhotic patients who needed anti-portal hypertension therapy with severe portal hypertension more than 12 mmHg in HVPG. The diagnosis of liver cirrhosis was confirmed in 231 patients by liver biopsy and the presence of varices in the esophagogastroduodenoscopy, laboratory data, or image studies, including ultrasonography and computer tomography (CT) scans, in the others. Patients who did not provide informed consent or had hepatocellular carcinoma, other malignancies within the past 3 years, severe hepatic failure (serum bilirubin level >5 mg/dL or hepatic encephalopathy), thrombosis in the inferior vena cava or hepatic or portal vein, uncontrolled infectious conditions (such as spontaneous bacterial peritonitis or sepsis), heart failure greater than NYHA class III, acute renal failure or severe chronic renal failure (eGFR <30 mL/min/1.73 m2), uncontrolled hypertension, pregnancy or lactation, or any other medical or psychiatric problems deemed to be unsuitable for clinical study were excluded. After all exclusions, 61 patients were ultimately enrolled in this study (Fig. 1). For 61 patients, the measurement of hepatic venous pressure gradient (HVPG) was undertaken with general basic serologic and radiologic tests. As a result, 8 patients who showed HVPG less than 12 mmHg were excluded and finally 53 patients were enrolled. Of 53 enrolled patients, the etiology of cirrhosis was classified 3 groups. 43 patients were alcoholic cause, 8 patients were B viral cause and 2 patients were mixed cause (alcohol+ B viral). And subjects with varix were 21 in monotherapy group, 26 in combination group. Subjects with variceal bleeding history were 1 in monotherapy group, 7 in combination group. Subjects with ascites were 14 in monotherapy group, 16 in combination group.

Flow chart of study. EF, ejection fraction; SBP, spontaneous bacterial peritonitis; EGD, esophagogastroduodenoscopy; HVPG, hepatic venous pressure gradient; NSBB, non-selective beta blocker.
The presented study was approved by the institutional review board (IRB) for human research at the Yonsei University Wonju Severance Christian Hospital and written informed consent for the treatment and examination was obtained in all patients.
Study design
This study was done prospectively as a randomized open-labeled controlled trial involving one center in Republic of Korea. The primary endpoint was to compare response rate (%) between two groups based on follow-up HVPG after a 3-month of treatment. The response was defined when HVPG was reduced more than 20% from baseline or to an absolute value of less than 12 mmHg. The study groups were composed of two depends on the anti-PH medication. One was combination group of candesartan and propranolol and the other was propranolol monotherapy group. Baseline and follow up HVPG after 3 months medication were measured in all enrolled patients. After measurement of baseline HVPG, patients were randomized into two groups by the pharmacy at Wonju Severance Christian Hospital using serially numbered sealed envelopes in batches that designated a patient to one of two treatments. Combination therapy of oral candesartan and propranolol was given to 26 patients, and monotherapy of propranolol was given to 27. In combination group, 8 mg of candesartan was given daily to all patients with propranolol. The initial dose of propranolol was 40 mg a day and it was elevated by additional 40mg every other day to a maximum dose of 360mg to reach 25% reduction from baseline heart rate or minimal heart rate of 55 beats per minute to both combination and monotherapy groups. If a patient was intolerable or had less than 55 beats of heart rate per minute or systolic blood pressure of less than 90mmHg, the dose of propranolol was gradually reduced to the level which was tolerable to the patient. If the abnormal response persisted despite of dose reduction, the drug was temporarily discontinued. When the symptoms and signs improved, the drug was reintroduced in a dose less than the dose that caused the abnormal response. If the response occurred again during dose modification, the patient was dropped from the study. After 3 months later 2 patients (1 for ingestion of alcohol, 1 for lost to follow up) in combination therapy group and 2 patients (1 for ingestion of alcohol, 1 for intolerance) in monotherapy group were withdrawn. Then 25 patients of combination therapy group and 24 patients of monotherapy group were completed study with follow up HVPG measurement and entered data analysis (Fig. 1).
Hepatic venous pressure gradient measurement
All enrolled patients underwent measurement of HVPG between the 7th-10thdays of admission. HVPG was measured after an overnight fast. The right HV was catheterized per cutaneously through the femoral vein, and pressure was recorded in both the wedged position and the free position with a 7-F balloon-tipped catheter (Arrow International, Erding, Germany). All measurements were performed at least in triplicate, and permanent tracings were obtained on a multichannel recorder. HVPG was determined by subtracting the free HV pressure from the wedged HV pressure.17,18,19,20 The coefficient of variation of HVPG measurement was 7%.
Statistical analysis
Categorical variables were expressed as proportions. Continuous variables were expressed as the median, range. Because of small numbers included patients in both, we used non-parametric analysis. To compare differences between two groups, continuous data were analyzed using Mann-Whitney U test, while categorical data were analyzed using the chi-square and Fisher exact tests. The Wilcoxon test was conducted to compare paired continuous variables within a group. P-value of less than 0.05 was taken to indicate a statistically significant difference. Statistical analysis was performed with IBM SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA).
RESULTS
General characteristics of subjects
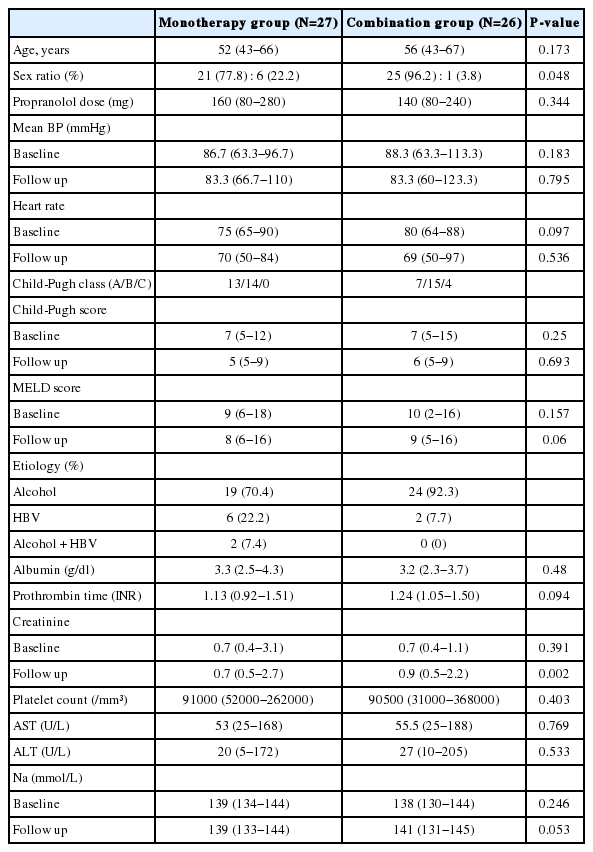
There was no difference in general characteristics between the combination and propranolol monotherapy group (Table 1). The mean dose of propranolol was 140 (80-240) mg in combination therapy group and 160 (80-280) mg in monotherapy group (P=0.344).
Treatment response between two groups
Response rates were 61.5 % (16 out of 26) in combined therapy group, and 55.6 %(15 out of 27) in propranolol monotherapy group (P=0.435). In combination group, the HVPG reduced significantly from 16 (12-28) mmHg to 13.5 (6-20) mmHg after 3 months treatment (P<0.001). The monotherapy group also showed significant reduction in HVPG from 17 (12-27) mmHg to 14 (7-25) mmHg (P<0.001) (Fig. 2). However, the reduced pressure by medication between the two groups had no significance difference (P=0.674). In addition, the relative pressure change rates by HVPG were 22.2 (-66.6-58.8)% in the monotherapy group and 21.8 (-18.7-60)% in the combination group, and there was no significance difference (P=0.783) (Fig. 3). Mean blood pressure and reduction in pulse rate also showed no statistical difference between the two groups. Child-Pugh and MELD score change also showed no difference between two groups (Table 2).
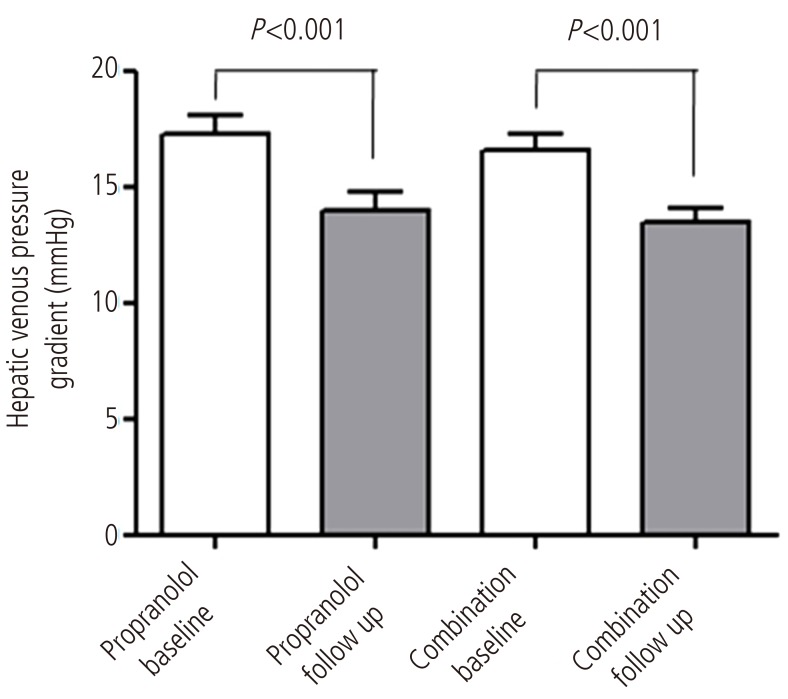
Comparison of the HVPG (mmHg) between baseline and 3 months after treatment in each group. The HVPG reduced significantly in both groups after 3 months of treatment (P<0.001). In the combination group, the HVPG reduced from 16 mmHg (range, 12-28 mmHg) to 13.5 mmHg (range, 6-20 mmHg) after 3 months of treatment with propranolol plus candesartan (P<0.001). In the propranolol monotherapy group, the HVPG reduced from 17 mmHg (range, 12-27 mmHg) to 14 mmHg (7-25 mmHg) after 3 months of treatment (P<0.001).
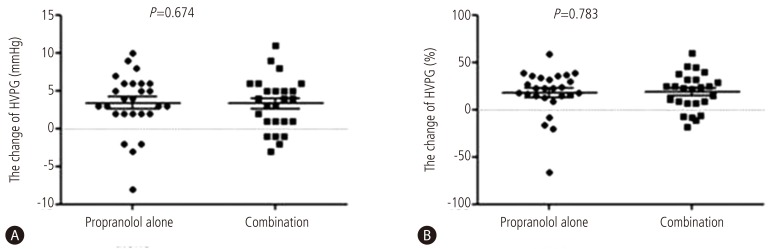
Comparison of the change in HVPG (mmHg) and in the change ratio of HVPG after 3 months of therapy to reduce portal hypertension. A Both treatment groups exhibited significant changes in the mean HVPG: by 3.5 mmHg (range, -3-11 mmHg) in the combination therapy group, and by 3 mmHg (range, -8-10 mmHg) in the monotherapy group, with no significant difference between the two groups (P=0.674). B The change ratio of HVPG was also significantly altered in both groups: by 6.6% (range, -26.6-33.4%) in the combination therapy group, and by 22.2% (range, -66.6-58.8%) in the monotherapy group, also with no significant difference between the groups (P=0.783).
Adverse reactions
In propranolol monotherapy group, there were two cases of orthostatic dizziness, two cases of generalized weakness, and a case of acute bradycardia. Incombined group, there were two cases of acute bradycardia, and two cases of orthostatic dizziness. The related symptoms were managed. In both groups there was no hepatic failure or AV block.
DISCUSSION
Prevention of variceal growing and bleeding is very crucial in managing and treating patients with cirrhosis. NSBB is the only generally recommended medication to reduce increased portal pressure, and lowers portal pressure mainly through attenuation of portal blood flow.21 The use of NSBB demonstrated a mean reduction in portal pressure of 15%,22and a satisfactory hemodynamic response was observed in about 58% of patients with cirrhosis.23 Moreover, about 15% of these patients were either hypersensitive to NSBB or unable to continue the treatment. In addition, NSBB is known to unable to prevent the occurrence of new varices.24
For this reason, a number of studies have been undertaken to develop a new medical therapy for portal hypertension. In this regard, the potential improvement in resistance to blood flow within the liver has increasingly drawn attention. IHR is composed of two components. One is static component by extracellular matrix deposition and structural changes of hepatic sinusoids. The other is relatively dynamic and reversible portion which is caused by sinusoidal twist and contraction by activated HSCs which play the key role in hepatic fibrosis.21,25 The former and the latter account for about 70-80% and 20-30% of total intrahepatic resistance, respectively. Therefore, theoretically, the inhibition of HSCs can alleviate PH by reducing IHR. Angiotensin II is the substance that has an ultimate effect in the RAA system, and is known to be involved in pathologic mechanism of increasing IHR in chronic liver diseases by inducing the contraction of HSCs.11
ARBs like irbesartan and losartan have been tested with the aim to reduce portal pressure in many clinical trials. Almost studies studied about the HVPG response rate with ARB monotherapy, however the results were controversial. Some studies showed benefit effect14,15 but other papers showed that ARB was not appropriate to clinical use because of hazard effects such as hypotension and renal impairment with26 or without portal pressure reduction.14,15,26,27 As mentioned above, in the all previous study ARB was applied alone, and the effective dose was too high and resulted many complications such as renal impairment. Therefore in the present study we tried to compare ARB and NSBB combination therapy with NSBB monotherapy for the first time. We aimed to investigate whether the combination therapy with just safe range dose of ARB and standard dose of NSBB could reduce both intrahepatic resistance and portal blood flow and show better HVPG response rate compared to NSBB monotherapy. Therefore, we fixed the dose of candesartan as daily 8 mg to prevent renal complication, and we added and elevated propranolol dose depend on patients hemodynamic condition in combination group.
As a result, the rates and features of side effects were similar between two groups. The biochemical examination, liver and renal functions, electrolyte balance as well as blood cell contents did not change in either group. In addition, no serious side effects, including renal failure or hepatic decompensation, were noted. Mean blood pressure changed mildly but significantly between the initiation and completion of the study. However, no patient had a hypotensive reaction that required cessation of medication during treatment, which is indicative of good tolerance. Candesartan administration was deemed to be safe in terms of circulatory hemodynamics as well as liver function in patients with liver cirrhosis with PH.
However, the addition of candesartan to propranolol did not show additional effect in the lowering of PH. The result could be explained by several reasons. First of all, we need to think about the dose of candesartan. Unlike propranolol, as mentioned above, to avoid renal complication ARB dose was fixed as same dose in all enrolled patients. We previously reported the beneficial effects of ARB on hepatic fibrosis in a rat model and clinical trial.10,28 Of the ARBs, candesartan is a potent long-lasting selective agent, which can produce strong antagonism because of its tight binding and slow dissociation from AT1-Rs. Therefore, its doses (8 mg once a day) were lower than those of other antagonists29,30,31 but it showed enough effect in anti-fibrosis. We predicted its dose would be also enough to the intrahepatic resistance and PH. However, the result between anti-fibrotic effect and anti-portal hypertensive effect was different and there is a possibility that the dose of ARB was not enough to reduce the HSC contractility and intrahepatic resistance. The pharmacokinetic individuality of candesartan also might affect the results.
In the line of above reason, per oral administration of ARB to the level that reduces the intrahepatic resistance probably needs very high dose however it is impossible in clinical practice because of risk of too severe hypotension and serious renal problem. It suggests the limitation of ARB in the application of PH treatment.
In addition, ARB absorbed through gastrointestinal tract can act with so many AT1-R in splanchnic organ before it arrives at portal blood flow into liver and it can also induce to unexpected direction in hemodynamics and aggravation of hyperdynamic circulation. Therefore, when we put together all above thing, special delivery system to carry ARBs into the liver at an optimum level needs to be developed in order to ensure desirable effect of ARB by applying clinically desirable dose without other organs side effects.
This study has several limitations. First of all, this study was performed without statistical design for proper sample size and we cannot deny the possibility that this point attenuated the statistical results and power. In addition, the fixation of ARB dose can be another limitation of this study to investigate the effect of combination therapy.
In conclusion, in the present study, the combined therapy of candesartan, an ARB and propranolol, showed significant decrease in HVPG after 3 months therapy; however the response rate was not better than propranolol monotherapy. ARB additional therapy to NSBB seems less effective within the traditional clinical dose and administration method and further well designed study for variable ARB's dose and clinical situation need to be followed.
Acknowledgement
This study was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. 100054).
Notes
The authors have no conflicts to disclose.
Abbreviations
ARB
angiotensin receptor blocker
AT1-R
angiotensin II type 1 receptor
HVPG
hepatic venous pressure gradient
HSC
hepatic stellated cells
IHR
intrahepatic résistance
NSBB
non-selective beta blocker
PH
portal hypertension
RAA
renin-angiotensin-aldosterone