Predictive factors of contrast-enhanced ultrasonography for the response to transarterial chemoembolization in hepatocellular carcinoma
Article information
Abstract
Background/Aims
The predictive role of contrast-enhanced ultrasonography (CEUS) before performing transarterial chemoembolization (TACE) has not been determined. We assessed the possible predictive factors of CEUS for the response to TACE.
Methods
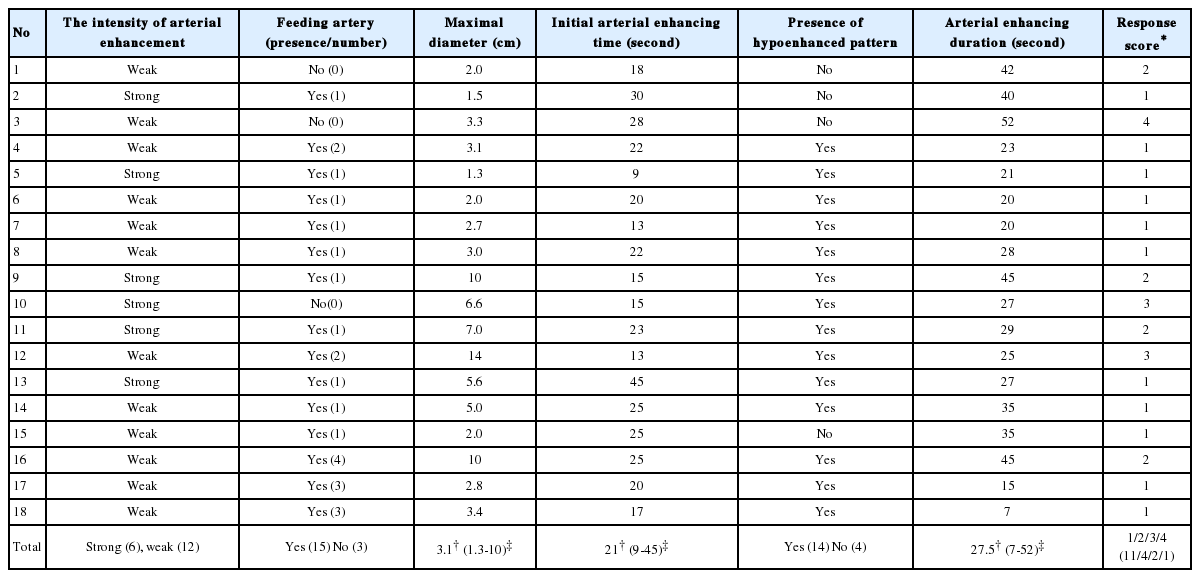
Seventeen patients with 18 hepatocellular carcinoma (HCC) underwent TACE. All of the tumors were studied with CEUS before TACE using a second-generation ultrasound contrast agent (SonoVue®, Bracco, Milan, Italy). The tumor response to TACE was classified with a score between 1 and 4 according to the remaining enhancing-tumor percentage based on modified response evaluation criteria in solid tumors (mRECIST): 1, enhancing tumor <25%; 2, 25%≤enhancing tumor<50%; 3, 50%≤enhancing tumor<75%; and 4, enhancing tumor≥75%). A score of 1 was defined as a "good response" to TACE. The predictive factors for the response to TACE were evaluated during CEUS based on the maximum tumor diameter, initial arterial enhancing time, arterial enhancing duration, intensity of arterial enhancement, presence of a hypoenhanced pattern, and the feeding artery to the tumor.
Results
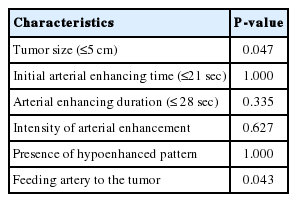
The median tumor size was 3.1 cm. The distribution of tumor response scores after TACE in all tumors was as follows: 1, n=11; 2, n=4; 3, n=2; and 4, n=1. Fifteen tumors showed feeding arteries. The presence of a feeding artery and the tumor size (≤5 cm) were the predictive factors for a good response (P=0.043 and P=0.047, respectively).
Conclusions
The presence of a feeding artery and a tumor size of less than 5 cm were the predictive factors for a good response of HCC to TACE on CEUS.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most frequently diagnosed cancer and the third cause of cancer-related mortality worldwide.12 Most patients with HCC are diagnosed late, and curative treatments cannot be applied. Additionally, high proportion of cases recur after curative therapy.3 Transarterial chemoembolization (TACE) is the most widely used primary treatment for unresectable HCC, and is recommended as first-line treatment for patients at intermediate stage of the disease by Barcelona Clinic Liver Cancer (BCLC) Staging System (B).4 HCC exhibits intense neoangiogenic activity during its progression.5 The rationale for TACE is that the intra-arterial infusion of a chemotherapeutic agent followed by embolization of the tumor-feeding artery will result in a strong cytotoxic and ischemic effect.67 Therefore, identification of the correct tumor-feeding artery is of definite importance on superselective (chemo) embolization. Successful subsegmental TACE in patients with HCC is related to a high degrees of tumor necrosis, low recurrence rates and prolonged survival.89 While, incorrect selection and subsequent TACE of a normal artery adjacent to the HCC will only result in damage of the normal liver parenchyma. So it is essential to evaluate tumor vascularization and distribution before TACE and during follow-up. Contrast-enhanced ultrasonography (CEUS) is now recognized as a useful imaging tool for the noninvasive diagnosis of small newly detected liver nodules during HCC surveillance and is also useful for guidance and assessment after loco-regional therapy of HCC.1011 Additionally low mechanical index (MI) real-time ultrasound in combination with ultrasound contrast agent (UCA) allows the real-time assessment of tumor vascularity and enhancement during the different vascular phases (arterial, portal venous, and delayed phases) with better temporal resolution than with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI).12 However, the predictive role of CEUS before TACE has not yet been elucidated. So in this study, we investigated for the first time the predictive factors for the response of TACE using CEUS.
MATERIALS AND METHODS
Patients and HCCs
Consecutive 17 patients (13 men, 4 women; mean age: 60±12.1 years) with HCC who underwent CEUS before TACE and were evaluated for the response of TACE between April 2010 and March 2012 at Soonchunhyang University Hospital, Seoul, Korea were investigated retrospectively. A total of 18 target HCCs from 17 patients were assessed for the possible predictive factors on CEUS to the response of TACE. All target lesions were clearly characterized in abdominal US (ultrasonography) and successfully identified with CEUS. Patients were diagnosed with HCC by percutaneous liver biopsy or by the presentation of typical HCC radiological features of a hypervascular lesion arising in the setting of cirrhosis with arterial phase enhancement and portal venous phase washout and/or an elevated serum alpha-fetoprotein level using Korean Liver Cancer Study Group-National Cancer Center (KLCSG-NCC) practice guidelines in 2014.13 HCC was staged by the Modified Union for International Cancer Control (mUICC) stage.14 The decision to offer TACE to patients with HCC was made by a multidisciplinary medical team including hepatologists, surgeons, medical oncologists, therapeutic and interventional radiologists. Candidacy for TACE was established using KLCSG-NCC practice guidelines in 2014.13
CEUS
All target HCCs were studied with CEUS before TACE. The mean time interval between CEUS and TACE was 8.6±7.6 days. A 2nd generation echo-enhancer SonoVue®, a low MI technique and software dedicated to harmonic imaging in abdominal US were used. To investigate the predictive factors of CEUS for the response of TACE, maximal diameter of tumor, initial arterial enhancing time, arterial enhancing duration, the intensity of arterial enhancement (strong: whole tumor enhancement with high echo intensity, weak: whole or partial tumor enhancement with low echo intensity), presence of hypoenhanced pattern, and feeding artery to the tumor were evaluated during CEUS. All CEUS studies were performed by two experienced hepatologists.
TACE
A 5-French catheter was inserted into the common femoral artery and angiographic survey of the celiac and superior mesenteric arteries was performed. Digital subtraction angiography (DSA) was also performed after selective catheterization of the proper, right and/or left hepatic arteries. Vessels supplying one or two hepatic segments were selected with a coaxially placed microcatheter (Masters Parkway Soft, ASAHI, Aichi, Japan). Chemoembolization was performed with adriamycin mixed with lipiodol, which was infused into the probable tumor feeder through segmental/subsegmental hepatic artery. The administered doses of chemotherapeutic agents were adjusted in patients with tumor size (adriamycin 10 mg per 1 cm diameter of tumor), liver or renal dysfunction, leukopenia, and thrombocytopenia. Administration of the emulsion was followed by embolization with a slurry of gelatin sponge (Cutanplast, Mascia Brunelli Spa, Milano, Italy; Cali-Gel, Alicon, Zhejiang, China) until stasis was achieved. TACE with drug-eluting beads were performed for two patients with HCC. The chemoembolic mixture consisted of a suspension of preloaded, drug-eluting microspheres (DC Beads Biocompatibles Ltd, Surrey, UK). DC Beads were preloaded with doxorubicin (Adriblastina, Pfizer Italia S.r.L., Nerviano, Milano, Italy) at a dose of 25-37.5 mg drug/ml of hydrated beads. Each patient received 2-4 mL of DC Beads (diameters: 100-300 µm and 300-500 µm).
Post-TACE response assessment
We assessed post-TACE response based on modified response evaluation criteria in solid tumors (mRECIST)1516 tumor necrosis quantification on lipiodol contrast-enhanced CT which was underwent approximately 4 weeks after TACE. The mean time interval between TACE and lipiodol contrast-enhanced CT was 32±10.5 days. After TACE, tumor response was classified with a score between 1 and 4 according to the percentage of remaining enhancing tumor portion (score 1, remaining enhancing lesion <25%; score 2, 25%≤enhancing lesion<50%; score 3, 50%≤enhancing lesion<75%; score 4, enhancing lesion≥75%). Score 1 was defined as "good response" after TACE. We analyzed the correlation between post-TACE response score and tumor size, initial arterial enhancing time, arterial enhancing duration, intensity of arterial enhancement, presence of hypoenhanced pattern, and feeding artery to the tumor.
Statistics
Numerical data were expressed as mean value with ± standard deviation or median value with range. The correlation between post-TACE response score and tumor size, initial arterial enhancing time, arterial enhancing duration, intensity of arterial enhancement, presence of hypoenhanced pattern, and feeding artery to the tumor were analyzed using Fisher's exact test. A P-value of <0.05 was considered significant. All statistical tests were 2-sided and performed with SPSS ver. 18.0 (SPSS Inc.,Chicago, IL, USA).
RESULTS
The demographics such as gender, age of the enrolled patient population and target HCC lesion characteristics such as causes, segmental distribution, mUICC stage, and Child-Pugh score are shown in Table 1. Features of target HCCs according to possible predictors on CEUS before TACE and post-TACE response scores were evaluated on the individual target HCC (Table 2). Median size of tumor was 3.1 cm (range 1.3-14 cm) and median initial arterial enhancing time was 21 seconds. Median arterial enhancing duration was 27.5 seconds and six tumors showed strong arterial enhancement. The presence of hypoenhancement pattern were 14 tumors. Fifteen tumors showed feeding arteries. The number of tumor response score after TACE in all tumors were 11 tumors with score 1, 4 with score 2, 2 with score 3, and 1 with score 4. A tumor with score 1 is noted in Figure 1. In predicting good response after TACE, initial arterial enhancing time, arterial enhancing duration, intensity of arterial enhancement, and hypoenhanced pattern did not show any significance. Presence of a feeding artery and tumor size (≤5 cm) were predictive factors for good response, respectively (P=0.043, P=0.047) (Table 3).
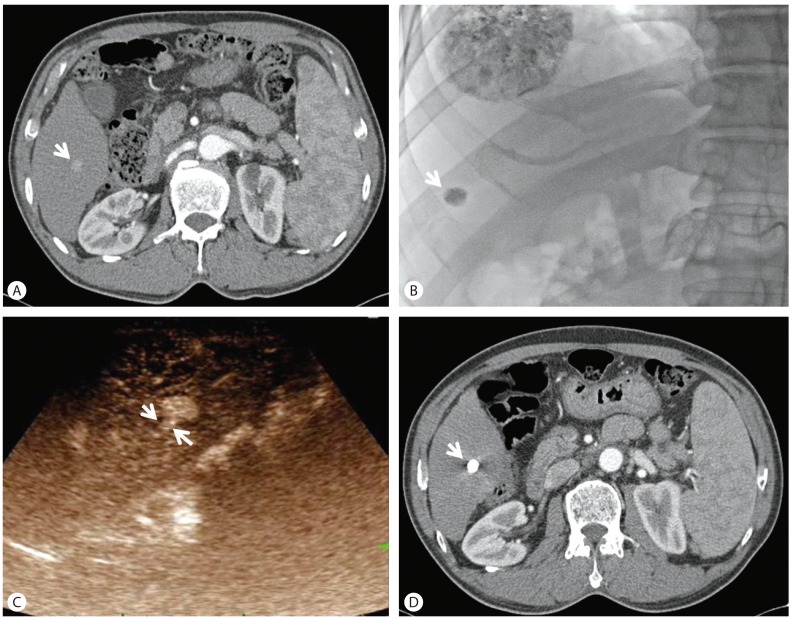
A tumor with a score of 1 after TACE. (A) An arrow indicates an enhancing HCC at arterial phase. (B) A tumor after TACE is indicated by an arrow. (C) Two arrows indicate the feeding artery to HCC in arterial phase of CEUS. (D) A compact lipiodol uptake after TACE is indicated by an arrow. TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; CEUS, contrast-enhanced ultrasonography.
DISCUSSION
CEUS is a flexible and versatile tool to many hepatologists, playing an important role in therapeutic planning, interventional guidance and post-treatment evaluation. We investigated the predictive factors for the response of TACE using CEUS for the first time.
And we used the low MI real-time ultrasound in combination with second generation UCA SonoVue® and software dedicated to harmonic imaging for the evaluation of predictive factors with the following reasons. First, physically the generation of harmonics in CEUS is related to nonlinearly (squared relation between second harmonic and applied acoustic pressure) to fundamental frequency energy.17 Imaging in the harmonic range will eliminate much of the near-filed artifacts such as reverberation and scattering. So it allows intravascular image of high-resolution during entire vascularphase.18 Second, current UCAs are typically microbubbles encapsulated by a stabilizing shell such as albumin, polymer, or phospholipid. Microbubbles are smaller than red blood cells (up to 7 µm in diameter), so they can easily pass through the capillary beds. On the contrary, they are too big to escape through the vascular endothelial pore unlike contrast agent of CT or MRI, thereby acting as excellent blood pool tracers.19 Third, at low acoustic peak pressure levels (low MI), microbubbles don't burst but oscillate (due to blood circulation) during enough vascularphase unlike first-generation UCA using high MI.20 Therefore, the above imaging tool facilitates real-time vascularphase (i.e., in the arterial, portal venous, and delayed phases) imaging of high-resolution for several minutes.2122
CEUS has another several advantages to assess the predictive factors for the response of TACE comparing with contrast-enhanced CT and MRI. CEUS is real-time imaging, whereas both contrast-enhanced CT and MRI are static imaging during each of the phases of enhancement. Some HCCs show transient hypervascularity in the arterial phase of real time CEUS, however they do not show enhancement in contrast-enhanced CT and MRI.2324 So we can infer that CEUS is more effective for showing feeding artery than contrast-enhanced CT and MRI. Additionally, CEUS can be used without risk of nephrotoxicity or requirement for ionizing radiation.
However, CEUS has disadvantages against contrast-enhanced CT and MRI. It cannot exhibit comprehensive assessment of the whole liver parenchyma during the short duration of the arterial phase.25 Another limitation is that like basic ultrasound techniques, CEUS can't get clear imaging in patients with a poor acoustic window including deep seated lesion.26 In addition, if multiple lesions or very large tumor are present, the lesions or the whole tumor can't be captured on single plane. Therefore, contrast-enhanced CT or MRI is effective for comprehensive assessment of the whole liver before TACE, but CEUS can be effective for prediction of the response of the target HCC.
According to the result of this study, initial arterial enhancing time, arterial enhancing duration, intensity of arterial enhancement, and hypoenhanced pattern did not show any significance, whereas presence of a feeding artery and tumor size (≤5 cm) were predictive factors for good response, respectively.
Presence of a feeding artery was the significant predictor for good response of TACE and this result confirms that above all, identification of the correct tumor-feeding artery is of definite importance for the good response of TACE.89 Kwan et al27 verified that the presence of a tumor-feeding artery greater than 0.9 mm in diameter on the pre-TACE visceral angiogram is a significant favorable predictor of >90% TACE-induced tumor necrosis. The correlation between definite presence of feeding artery on CEUS and definite presence of tumor-feeding artery on pre-TACE visceral angiogram or DSA needs to be further investigated. And if the concordance can be verified, further research will be required for confirm the efficacy of contrast-enhanced ultrasound with intraarterial administration of UCA (i.a CEUS) guidance, which distinguish and help identify correct tumor-feeding artery in HCC with a complex vascular supply, or in small and hypovascular HCC, with little or no tumor blush on DSA during TACE.28 In this study, tumor size (≤5 cm) was also significant factor for favorable response. In terms of tumor size, the large size tumor generally has multiple feeding arteries and it seems that it is more difficult to embolize all feeding arteries and make large tumor be necrotized.
But, this study has several limitations. First, large HCC with multiple feeding arteries might be not captured on single plane at the same time during arterial enhancement. Second, there was a little bit of range for time interval between CEUS and TACE, and also the time interval between TACE and lipiodol contrast-enhanced CT. Third, the retrospective nature and small number of target HCCs may be potential limitations of this study. So to validate the result of this study, there are need for large sized population and prospective design.
In conclusion, this study showed real-time imaging of CEUS can effectively predict the response of TACE for target HCC, and the presence of a feeding artery and tumor size less than 5 cm were the predictive factors for the good response of TACE on CEUS.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
CEUS
contrast-enhanced ultrasonography
CT
computed tomography
DSA
digital subtraction angiography
HCC
hepatocellular carcinoma
KLCSG-NCC
Korean Liver Cancer Study Group-National Cancer Center
MI
mechanical index
mUICC
Modified Union for International Cancer Control
MRI
magnetic resonance imaging
TACE
transarterial chemoembolization
US
ultrasonography
UCA
ultrasound contrast agent