| Clin Mol Hepatol > Volume 21(1); 2015 > Article |
ABSTRACT
Background/Aims
Silibinin, the main component of silymarin, is used as a hepatoprotectant and exhibits anticancer effects against various cancer cells. This study evaluated the effects of a combination of silibinin with either gefitinib or sorafenib on hepatocellular carcinoma (HCC) cells.
Methods
Several different human HCC cell lines were used to test the growth-inhibiting effects and cell toxicity of silibinin both alone and in combination with either gefitinib or sorafenib. The cell viability and growth inhibition were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, trypan blue staining, and a colony-forming assay. Furthermore, changes in epidermal growth factor receptor (EGFR)-related signals were evaluated by Western blot analysis.
Results
Gefitinib, sorafenib, and silibinin individually exhibited dose-dependent antiproliferative effects on HCC cells. Combined treatment with silibinin enhanced the gefitinib-induced growth-inhibiting effects in some HCC cell lines. The combination effect of gefitinib and silibinin was synergistic in the SNU761 cell line, but was only additive in the Huh-BAT cell line. The combination effect may be attributable to inhibition of EGFR-dependent Akt signaling. Enhanced growth-inhibiting effects were also observed in HCC cells treated with a combination of sorafenib and silibinin.
Liver cancer is the sixth most common neoplasm in the world, and its poor prognosis makes it the third leading cause of cancer-related mortality.1 Because of early angioinvasion and metastasis, only a small proportion of patients with hepatocellular carcinoma (HCC) receives surgical resection or liver transplantation as a curative treatment.2 Except for sorafenib, a multikinase inhibitor, there are no effective systemic treatments of HCC. Two large phase III randomized controlled trials showed that the median overall survival was 10.7 months3 and 6.5 months4 in a sorafenib-treated group. Therefore, sorafenib has been used as a salvage therapy for advanced HCC. However, in the treatment of HCC, sorafenib alone has demonstrated insufficient responses and has resulted in several adverse effects, such as hand foot skin reaction, diarrhea, and jaundice.3,4 Recently, there are several studies that address the multidisciplinary management of HCC, which includes sorafenib treatment,5,6,7,8,9 to improve overall survival as well as drug combination therapy based on sorafenib.10,11,12 Unfortunately, there has been no definite combination treatment that improves the overall survival of HCC patients.
Milk thistle (Silybum marianum) has been used widely as an herbal remedy for liver disease. Milk thistle extract is composed almost completely of silymarin, and silibinin (silybin) is the major active compound of silymarin.13,14 Many studies have revealed the antitumor effects of silibinin on multiple cancer cells, such as prostate,15,16,17,18 colon,19,20 skin,21,22 bladder,23,24,25 and lung cancers,26 Moreover, Varghese et al. and Lah et al. identified the efficacy of silibinin in HCC cells.27,28 Recently, Rho et al reported that combined treatment with silibinin and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) overcame drug resistance caused by the T790M mutation in non-small cell lung cancer cell lines.29 These results demonstrated the combined synergistic effect of gefitinib and silibinin.
We investigated the efficacy of combined treatment with silibinin and either sorafenib or gefitinib on HCC cells and suggest a new strategy in the treatment of HCC.
To assess the combination effects, we compared the cell growth after combined treatment with silibinin and either gefitinib or sorafenib with the single treatment of each drug in the following human HCC cell lines: Huh7, HepG2, Huh-BAT, Hep3B, HepG2, PLC/PRF5, SNU387, SNU398, SNU449, SNU475, and SNU761.
We examined the cell growth by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, trypan blue staining, and a colony-forming assay then determined alterations in intracellular signals using Western blot analysis.
The human HCC lines (Hep3B, HepG2, Huh7, PLC/PRF5, SNU387, SNU398, SNU449, SNU475, and SNU761) were purchased from the Korean Cell Line Bank. Huh-BAT cells were obtained from the Liver Research Institute, Seoul National University. Gefitinib was kindly provided by AstraZeneca Korea. Sorafenib and silibinin were purchased from Selleck Chemicals (Houston, TX, USA) and Sigma-Aldrich Co. (St. Louis, MO, USA), respectively.
The human HCC cell lines were cultured in DMEM, MEM, and RPMI media supplemented with 10% fetal bovine serum and 100 U/mL penicillin in 5% CO2 at 37℃.
Cells were plated in 96-well plates at a density of 5,000 cells/well and cultured overnight. Then, the cells were exposed to varying concentrations of either gefitinib or sorafenib or silibinin for 72 h. Subsequently, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was added to the media containing the various drugs; after incubating for 3 h, a 10% SDS solution was added to the samples. The samples were cultured for 24 h, and the color absorbance was read at a wavelength of 595 nm.
Cells were plated in 60-mm plates at a density of 1,000 cells/well and cultured overnight. Then, the cells were treated with either gefitinib or sorafenib and/or silibinin for 72 h. Thereafter, the cells were incubated in drug-free media for 2 weeks and then stained using methylene blue. The colonies were photographed and then counted.
Cells were plated in 60-mm plates at a density of 1,000 cells/well and cultured overnight. Then, the cells were treated with either gefitinib or sorafenib and/or silibinin for 72 h. Thereafter, the cells were incubated in drug-free media for 10 days and then stained using trypan blue to count cell numbers.
Combined at a constant ratio, gefitinib or sorafenib:silibinin (1:100 µM) were measured by an MTT assay. CI plots were computationally generated by CalcuSyn software (Biosoft, Cambridge, UK)
Cell lysates were prepared in non-denaturing lysis buffer (20 mmol/L Tris-HCl; pH 7.4; 150 mmol/L NaCl; 1 mmol/L EDTA; 1 mmol/L EGTA; 1 mmol/L ß-glycerophosphate; 1 mmol/L sodium vanadate; 1 µg/mL leupeptin; 1 mmol/L PMSF). The protein was collected after centrifugation at 13,000 × g for 10 min. The resulting supernatant was separated on a 10% SDS-PAGE gel and transferred to Immobilon-P membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat skim milk-PBS-T (137 mmol/L NaCl; 2.7 mmol/L KCl; 10 mmol/L phosphate buffer; 0.01% Tween-20) for 1 hr at room temperature before being incubated overnight with a primary antibody against p-EGFR, EGFR, IGF-1R, p-Akt, Akt and Erk (all from Santa Cruz Biotechnolongy [Santa Cruz, CA, USA]), and p-IGF-1R and p-Erk (from Cell Signaling Technology [Beverly, MA, USA]), and β-actin (from Sigma-Aldrich [St. Louis, MO, USA]). Then, the membrane was washed three times in PBS-T for 5 min and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h and washed three times in PBS-T for 10 min. After washing, the membrane was developed using chemiluminescence and exposed to X-ray film to visualize the protein bands.
Cell viability was evaluated by MTT assay in the following gefitinib- or silibinin-treated HCC cell lines: Huh7, Huh-BAT, HepG2, SNU475, and SNU761. Gefitinib treatment resulted in the dose-dependent inhibition of Huh7, Huh-BAT, HepG2, and SNU761 cell growth (Fig. 1A). Silibinin treatment also significantly inhibited the cell growth of Huh7, Huh-BAT, and SNU761 cells; however, the cell growth of HepG2 and SNU475 cells increased with low doses of silibinin and decreased in high doses of silibinin.
The combined-treatment effect of gefitinib and silibinin is displayed in Figure 1B. The combined-treatment effects of gefitinib and silibinin were assessed in SNU475, SNU761, Huh7, Huh-BAT, and HepG2 cells and demonstrated a significant inhibiting effect in SNU761 and Huh-BAT cells. The CI in the SNU761 cells was below 1, which indicates a synergistic effect of gefitinib and silibinin combination treatment. However, in Huh-BAT cells, the CI was below 1 at some doses, which indicates an additive effect rather than a synergistic effect (Fig. 1C and 1D).
In the SNU761 and Huh-BAT cell lines, the cell numbers were decreased more in the combined treatment (gefitinib and silibinin) compared with the single treatment (gefitinib or silibinin) (Fig. 2A and 2B). In addition, in the colony-forming assay, the colony numbers were decreased more in the combination treatment (gefitinib and silibinin) compared with the single treatment (gefitinib or silibinin) (Fig. 2C and 2D). These results established that the combined-treatment effects of gefitinib and silibinin produce significant inhibition of cellular growth in HCC cells.
To clarify the mechanism of the anti-proliferative effects, the activity of EGFR, IGF1R, and the downstream molecules of IGF1R were tested. The activity of IGF1R and IGF1R-related molecules were not altered, but the activity of EGFR was decreased in both SNU761 and Huh-BAT cells. In SNU761 cell lines, the activity of Akt, the downstream signal of the EGFR pathway, was decreased, but in Huh-BAT cell lines did not. These results are illustrated in Figure 2E.
To confirm the anticancer effect of the multikinase inhibitor sorafenib on HCC, we examined the cell viability of various HCC cells using an MTT assay. In all of the HCC cell lines tested, sorafenib inhibited cell growth in a dose-dependent manner. The half-maximal inhibiting concentrations (IC50) were approximately 1.77-2.64 µM in Huh7, Huh-BAT, HepG2, Hep3B, and PLC/PRF5 cells, which indicates that the anticancer effects of sorafenib on HCC are effective at a relative low dose; yet, the IC50 in the SNU387, SNU398, SNU475, and SNU761 cells were approximately 8.31-13.31 µM. The inhibiting effects of sorafenib were dependent on each HCC cell line (Fig. 3A).
The combination of silibinin and sorafenib demonstrated an additional inhibiting effect in SNU761, Huh7, PLC/PFR and Huh-BAT cells. The CIs indicated a synergistic effect of combined silibinin and sorafenib treatment at some doses. Cell growth and CIs are displayed in Figure 3B.
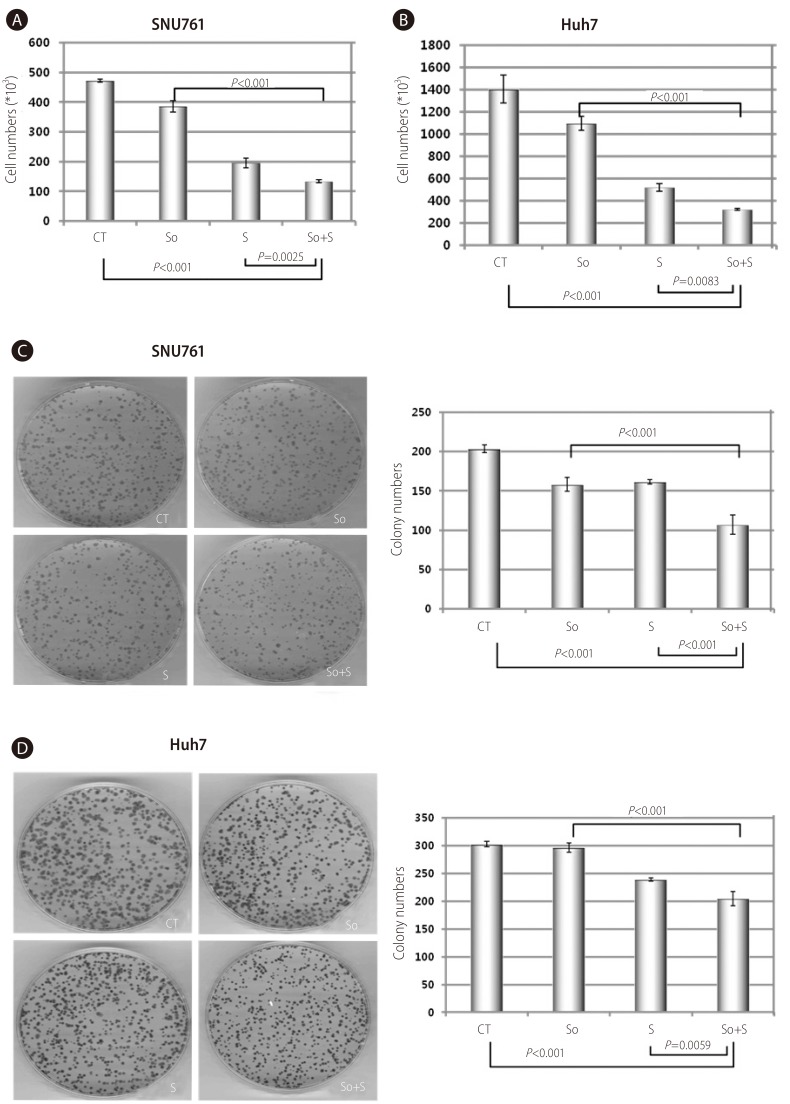
In SNU761 and Huh7 HCC cell lines, the cell numbers were more decreased with combined treatment than the single treatment of sorafenib or silibinin (Fig. 4A and 4B). The enhanced growth-inhibiting effect of combination treatment (sorafenib and silibinin) was confirmed again in colony forming assay by reduced colony numbers in combined treatment of sorafenib and silibinin (Fig. 4C and 4D).
HCC has a poor prognosis due to early vascular invasion, rapid growth rate, underlying chronic hepatitis or hepatic cirrhosis. Furthermore many HCC patients are diagnosed at advanced stage, so they cannot receive curative therapy.30,31 Sorafenib is approved as the standard of palliative therapy in patients with advanced HCC, but the clinical benefits have been limited.32 Therefore, the development of more effective and less hepatotoxic treatments with fewer side effects is necessary.
Empirically, silibinin has been used as a hepatoprotectant for more than 2000 years. Many studies have established the anti-cancer effect of silibinin against various cancers.15,16,17,18,19,20,21,22,23,24,25,26 The anti-tumor effect exhibited by silibinin is facilitated by the inhibition of EGFR activation, which is the first step of the signaling pathway of cell growth associated with tumor cell growth.19,20,25,27,28,33,34,35,36,37,38 Silibinin induces apoptosis via the downregulation of CDK expression, which regulates cell cycles, and the increased expression of p21 and p27, which inhibits CDK and arrests the cell cycle at G1 and G2.25,27,37 It was reported that the mechanism of EGFR-activation inhibition occurred via interruptions of ligand synthesis37, ligand binding39, and receptor dimerization29. Because silibinin is lipophilic, it can easily move on cell membranes and directly insert into membrane lipids and can have multiple actions. Moreover, silibinin suppresses angiogenesis and metastasis.34,40
This study investigated the effect of target agents combined with silibinin, which inhibits EGFR activation and regulates cellular growth factors. We obtained results by testing the target agents on many human HCC cell lines. Sorafenib, gefitinib, and silibinin could reduce the cell growth of many HCC cell lines. The combined treatment of gefitinib and silibinin significantly suppressed cell growth in some HCC cell lines, such as SNU761 and Huh-BAT cells, compared with gefitinib treatment alone. These results are attributed to alterations in the intracellular EGFR signaling pathway via the downregulation of Akt signaling. In some HCC cell lines, the combined treatment with silibinin and sorafenib significantly attenuated the cell growth compared with sorafenib treatment alone.
Varghese et al. and Lah et al. reported that silibinin significantly reduced the cell growth of many HCC cell lines.27,28 They showed the inhibiting effect of silibinin in a dose-dependent manner. In our study, we observed similar results. Moreover, silibinin has other, more common effects, such as antioxidant, anti-inflammatory, anti-fibrotic, and metabolic. Silibinin may offer protection from acute liver damage; therefore, many clinicians prescribe silibinin to prevent severe liver injury and to resolve liver damage. Additionally, silibinin is used for the treatment of chronic liver diseases, such as chronic hepatitis C and alcoholic hepatitis. In our study, we observed that the cell viability of the HepG2 and SNU475 cells was increased when treated with the low dose of silibinin. These results may explain the reasons for silibinin use in liver damage. While, silibinin showed anticancer effects in most of the HCC cell lines at a dose over 100 µM. Recently, Flaig et al employed large doses (2.5-20 g) of silibin-phosphatidylcholine.41 When treated with over 15 g of silibin-phosphatidylcholine, certain patients showed only asymptomatic hyperbilirubinemia; however, in all of the patients, hyperbilirubinemia was reversed after the discontinuation of silibin-phosphatidylcholine. This study provided evidence that silibinin can be administered at high doses. However, further investigations are warranted to investigate whether higher doses can induce a higher blood concentration and whether there is a relationship between the blood concentration and the tissue concentration of silibinin.
Gefitinib, an EGFR-TKI, is a potent anticancer agent on EGFR positive non-small cell lung cancer, especially adenocarcinoma.42 Some reports have stated that EGFRs are expressed frequently in HCC.43,44 Hopfner et al. described gefitinib-induced growth inhibition, apoptosis, and cell cycle arrest in HCC cells.45 Additionally, in our study, gefitinib reduced cell growth in each HCC cell line, except in SNU475 cells, in dose-dependent manner. Moreover, the combined treatment of HCC cells with silibinin and gefitinib showed a synergistic effect at all concentrations tested in SNU761 cells and at some concentrations tested in Huh-BAT cells; other HCC cell lines showed a significant reduction of cell growth at higher concentrations of silibinin and gefitinib treatment. In the trypan blue staining assay and the colony-forming assay, cell numbers and colony numbers were more decreased in HCC cell lines exposed to the combined treatment compared with the single treatment of gefitinib or silibinin. Furthermore, Western blot analysis revealed the decreased activity of EGFR and Akt in SNU761 cells. However, in Huh-BAT cells that demonstrated a synergistic effect of the combination treatment at partial concentrations, the activity of EGFR was declined, but the activity of Akt was not declined. These different effects indicate the heterogeneity of HCC in each HCC cell line.
Silibinin is a safe drug with a few mild adverse effects, including gastrointestinal symptoms such as abdominal distention, indigestion, nausea, and diarrhea, which are all common and reversible. The effective anticancer dosage of silibinin, as stated previously, could be higher than the current dosages used; this issue needs to be addressed in future investigations.
Sorafenib, which is used widely as an effective target agent for HCC treatment, was shown to possess anti-proliferative effects in all of HCC cell lines tested, although the IC50 in each of the cell line was different. The combined effects of silibinin and sorafenib on the HCC cell lines tested indicated a synergistic effect in a dose-dependent manner. Many ongoing studies are addressing which drug is most effective when combined with sorafenib.46,47 However, there are not any drugs available. Although this study was performed in vitro, it is promising that silibinin produces a synergistic effect with target agents, especially sorafenib. We suggest that combination treatment with target agents and silibinin could be a useful approach for future HCC treatments.
Acknowledgements
This research was supported by a grant of the Radiological Translational Research Program, Korea Institute of Radiological and Medical Sciences (50351-2009) and the GlaxoSmithKline Research Fund of the Korean Association for the Study of the Liver.
REFERENCES
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 21296855.


2. Fenoglio L, Serraino C, Castagna E, Cardellicchio A, Pomero F, Grosso M, et al. Epidemiology, clinical-treatment patterns and outcome in 256 hepatocellular carcinoma cases. World J Gastroenterol 2013;19:3207-3216. 23745022.



3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390. 18650514.


4. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the asia-pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. 19095497.


5. Cohen GS, Black M. Multidisciplinary management of hepatocellular carcinoma: A model for therapy. J Multidiscip Healthc 2013;6:189-195. 23690690.



6. Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol 2011;29:3960-3967. 21911714.



7. Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol 2012;56:1336-1342. 22314421.


8. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-2127. 21664811.


9. Zhao JD, Liu J, Ren ZG, Gu K, Zhou ZH, Li WT, et al. Maintenance of sorafenib following combined therapy of three-dimensional conformal radiation therapy/intensity-modulated radiation therapy and transcatheter arterial chemoembolization in patients with locally advanced hepatocellular carcinoma: A phase I/II study. Radiat Oncol 2010;5:12. 20149262.



10. Morisaki T, Umebayashi M, Kiyota A, Koya N, Tanaka H, Onishi H, et al. Combining celecoxib with sorafenib synergistically inhibits hepatocellular carcinoma cells in vitro. Anticancer Res 2013;33:1387-1395. 23564777.

11. Kelley RK, Nimeiri HS, Munster PN, Vergo MT, Huang Y, Li CM, et al. Temsirolimus combined with sorafenib in hepatocellular carcinoma: A phase I dose-finding trial with pharmacokinetic and biomarker correlates. Ann Oncol 2013;24:1900-1907. 23519998.



12. Alsaied OA, Sangwan V, Banerjee S, Krosch TC, Chugh R, Saluja A, et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery 2014;156:270-279. 24953273.


13. Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: Why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 2007;6:110-119. 17548790.


14. Gazak R, Walterova D, Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr Med Chem 2007;14:315-338. 17305535.


15. Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev 2003;12:933-939. 14504208.

16. Tyagi A, Agarwal C, Agarwal R. Inhibition of retinoblastoma protein (rb) phosphorylation at serine sites and an increase in rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: Role in prostate cancer prevention. Mol Cancer Ther 2002;1:525-532. 12479270.

17. Deep G, Gangar SC, Rajamanickam S, Raina K, Gu M, Agarwal C, et al. Angiopreventive efficacy of pure flavonolignans from milk thistle extract against prostate cancer: Targeting VEGF-VEGFR signaling. PLoS One 2012;7:e34630. 22514647.



18. Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis 2007;28:2567-2574. 17916909.


19. Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res 2007;143:58-65. 17950073.


20. Sangeetha N, Felix AJ, Nalini N. Silibinin modulates biotransforming microbial enzymes and prevents 1,2-dimethylhydrazine-induced preneoplastic changes in experimental colon cancer. Eur J Cancer Prev 2009;18:385-394. 19654488.


21. Mohan S, Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin modulates UVB-induced apoptosis via mitochondrial proteins, caspases activation, and mitogen-activated protein kinase signaling in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun 2004;320:183-189. 15207719.


22. Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and akt signaling. Cancer Res 2004;64:6349-6356. 15342425.


23. Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis 2004;25:1711-1720. 15117815.


24. Tyagi AK, Agarwal C, Singh RP, Shroyer KR, Glode LM, Agarwal R. Silibinin down-regulates survivin protein and mRNA expression and causes caspases activation and apoptosis in human bladder transitional-cell papilloma RT4 cells. Biochem Biophys Res Commun 2003;312:1178-1184. 14651997.


25. Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res 2008;14:300-308. 18172282.


26. Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog 2004;40:143-149. 15224346.


27. Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res 2005;11:8441-8448. 16322307.


28. Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol 2007;13:5299-5305. 17879397.



29. Rho JK, Choi YJ, Jeon BS, Choi SJ, Cheon GJ, Woo SK, et al. Combined treatment with silibinin and epidermal growth factor receptor tyrosine kinase inhibitors overcomes drug resistance caused by T790M mutation. Mol Cancer Ther 2010;9:3233-3243. 21159609.


30. Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology 2012;55:476-482. 21953588.



31. El-Serag HB, Siegel AB, Davila JA, Shaib YH, Cayton-Woody M, Mc-Bride R, et al. Treatment and outcomes of treating of hepatocellular carcinoma among medicare recipients in the united states: A population-based study. J Hepatol 2006;44:158-166. 16290309.


32. Lee JK, Abou-Alfa GK. An update on clinical trials in the treatment of advanced hepatocellular carcinoma. J Clin Gastroenterol 2013;47(Suppl):S16-S19. 23751800.


33. Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol Int 2008;32:888-892. 18538589.


34. Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res 2008;68:6822-6830. 18701508.



35. Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res 2002;62:3063-3069. 12036915.

36. Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. P21 and P27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther 2007;6:2696-2707. 17938263.


37. Tyagi A, Sharma Y, Agarwal C, Agarwal R. Silibinin impairs constitutively active TGFalpha-EGFR autocrine loop in advanced human prostate carcinoma cells. Pharm Res 2008;25:2143-2150. 18253818.


38. Hannay JA, Yu D. Silibinin: A thorny therapeutic for EGF-R expressing tumors. Cancer Biol Ther 2003;2:532-533. 14614321.


39. Sharma Y, Agarwal C, Singh AK, Agarwal R. Inhibitory effect of silibinin on ligand binding to erbB1 and associated mitogenic signaling, growth, and DNA synthesis in advanced human prostate carcinoma cells. Mol Carcinog 2001;30:224-236. 11346885.


40. Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res 2008;68:2043-2050. 18339887.


41. Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 2007;25:139-146. 17077998.


42. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-957. 19692680.


43. Daveau M, Scotte M, Francois A, Coulouarn C, Ros G, Tallet Y, et al. Hepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinoma. Mol Carcinog 2003;36:130-141. 12619035.


44. Moon WS, Park HS, Yu KH, Park MY, Kim KR, Jang KY, et al. Expression of betacellulin and epidermal growth factor receptor in hepatocellular carcinoma: Implications for angiogenesis. Hum Pathol 2006;37:1324-1332. 16949929.


45. Hopfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherubl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol 2004;41:1008-1016. 15582135.


Figure 1
Growth-inhibiting effects of gefitinib and/or silibinin in hepatocellular carcinoma cells (HCCs). A and B: Huh7, Huh-BAT, HepG2, SNU475, and SNU761 HCC cell lines were cultured in medium containing 5% fetal bovine serum (FBS) and treated with gefitinib (A, left), silibinin (A, right), or the two in combination (B) for 72 hr. The resulting cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. C and D: The combination effects of gefitinib (1 µM) and silibinin (100 µM) in SNU761 (C) and Huh-BAT (D) cells were measured using the MTT assay. Combination index (CI) plots were generated using computer software; CI values of <1, 1, and >1 indicate synergism, an additive effect, and antagonism, respectively. G, gefitinib; S, silibinin; G+S, gefitinib+silibinin.
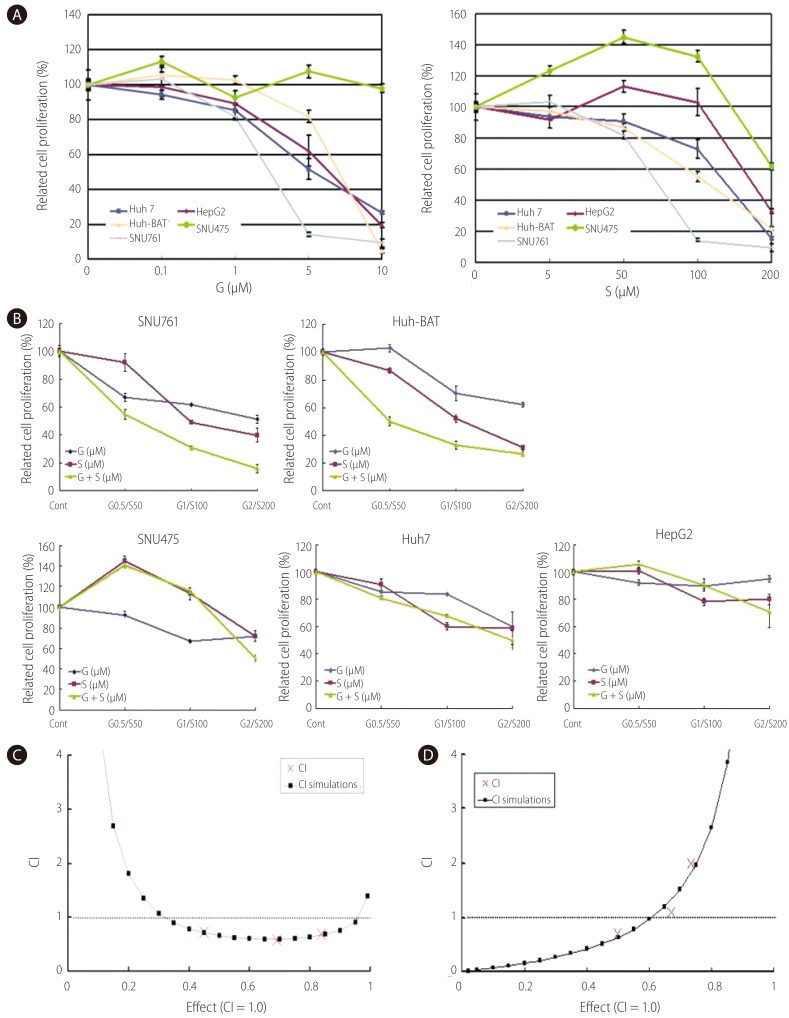
Figure 2
Combined treatment with gefitinib and silibinin inhibited cell proliferation through the inhibition of Akt signaling in HCC cells. A and B: SNU761 (A) and Huh-BAT (B) cell lines were treated with gefitinib, silibinin, or the two in combination for 72 hr, and viable cells were counted after staining with trypan blue. C and D: The combination effects of gefitinib and silibinin on HCC cells were evaluated using a colony-forming assay in SNU761 (C) and Huh-BAT (D) cells. E: SNU761 and Huh-BAT cells were treated with silibinin (100 µM) and gefitinib (1 µM) alone or in combination. After treating HCC cells with epidermal growth factor (EGF) or insulin-like growth factor 1, molecules of EGF receptor-related signaling were detected by Western blot analysis. CT, control; G, gefitinib (1 µM); S, silibinin (100 µM); G+S, gefitinib (1 µM)+silibinin (100 µM).

Figure 3
Growth inhibition of HCC cells by sorafenib alone or in combination with silibinin. A: Huh7, Huh-BAT, HepG2, Hep3B, PLC/PRF5, SNU387, SNU398, SNU449, SNU475, and SNU761 HCC cell lines were cultured in medium containing 5% FBS and treated with the indicated doses of sorafenib for 72 hr. The resulting cell viability was determined using the MTT assay. B: SNU761, Huh7, Huh-BAT, and PLC/PRF5 cell lines were cultured in medium containing 5% FBS and treated with the indicated amounts of sorafenib, silibinin, or the two in combination for 72 hr. The resulting cell viability was measured using the MTT assay. Cell viability curves (left) and CI plots (right) were generated using computer software. So, sorafenib; S, silibinin.
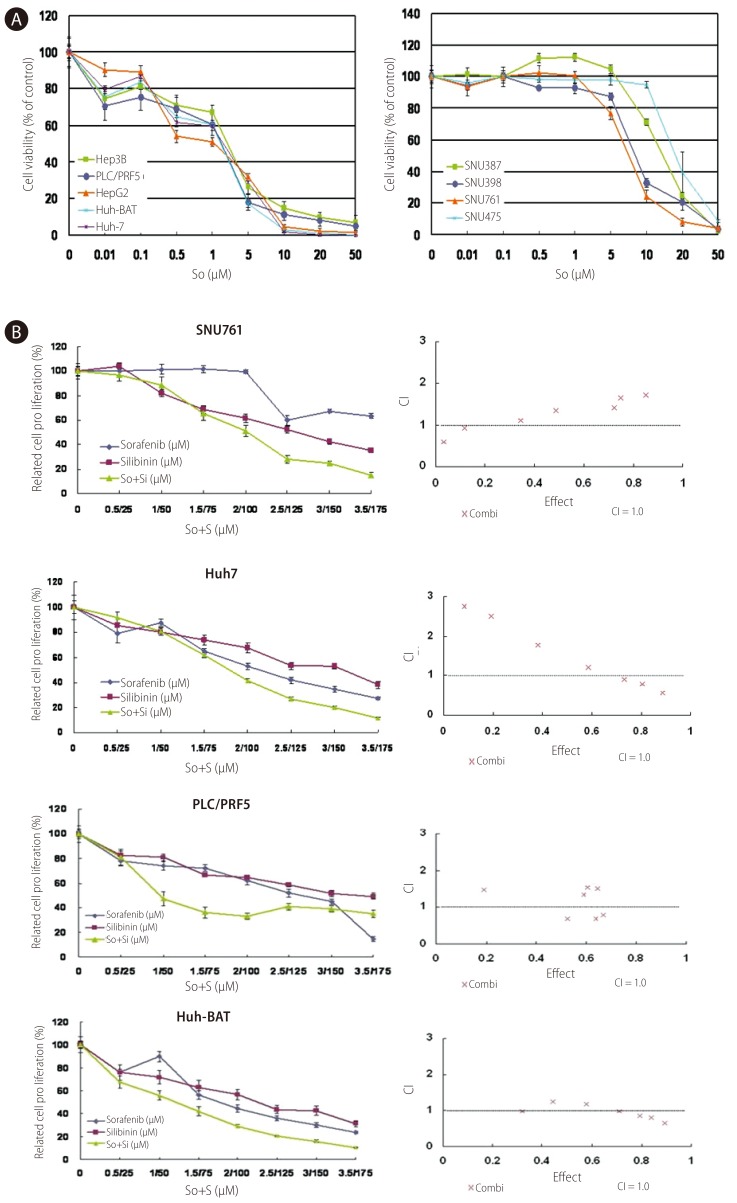
Figure 4
Combined treatment of sorafenib with silibinin conferred an additional growth-inhibiting effect in SNU761 and Huh7 HCC cell lines. A and B: SNU761 (A) and Huh-7 (B) cell lines were treated with sorafenib (2 µM), silibinin (100 µM), or a combination of the two for 72 hr, and viable cells were counted after staining with trypan blue. C and D: The combination effects of sorafenib (2 µM) and silibinin (100 µM) on SNU761 (C) and Huh-7 (D) HCC cells were evaluated using a colony-forming assay. CT, control; So, sorafenib (2 µM); S, silibinin (100 µM); So+S, sorafenib (2 µM)+silibinin (100 µM).
- TOOLS
-
METRICS

- Related articles
-
Which treatment modality should we choose for advanced hepatocellular carcinoma?2010 December;16(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



