INTRODUCTION
Cholangiocarcinomas arise from the epithelial cells of the bile ducts in the intrahepatic, perihilar, or distal (extrahepatic) biliary tree, and are exclusive of the gallbladder and ampulla of Vater. Common symptoms include pruritus, abdominal pain, weight loss, and fever. Intrahepatic cholangiocarcinomas originate peripherally within the intrahepatic ducts of the liver parenchyma, and form 5~10% of cholangiocarcinomas.1,2 Imaging studies are essential for the diagnosis of cholangiocarcinoma. Transabdominal ultrasound, contrast-enhanced, triple phase, and helical computed tomography, magnetic resonance cholangiopancreatography, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiopancreatography have been used to diagnose cholangiocarcinoma. Tumor markers such as carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA) can be useful in conjunction with biopsy or brushings to confirm the diagnosis. It is often difficult to make a definite diagnosis of cholangiocarcinoma preoperatively. Ultrasound or CT-guided percutaneous biopsy should be considered to confirm. 18F-fluoro-2-dexoy-D-glucose positron emission tomography is also useful for detecting lymph node metastasis, distant metastasis, and a recurrence of intrahepatic cholangiocarcinoma.3
A complete surgical resection is the only curative treatment for cholangiocarcinoma.2 Patients who have unresectable disease may need palliative therapies, such as surgical or endoscopic biliary drainage, chemotherapy, radiation therapy, or stenting.4 Intrahepatic cholangiocarcinomas often invade adjacent organs or metastasize to other visceral organs. However, skeletal muscle metastasis from cholangiocarcinoma is very rare. This report describes a case of skeletal muscle metastasis from an intrahepatic cholangiocarcinoma as the first distant metastasis that was identified on 2-[18F] fluoro-2-dexoy-D-glucose positron emission tomography (18F-FDG PET) during the initial work up.
CASE REPORT
A 45-year-old man was admitted to Department of Gastroenterology because of abdominal pain. An ultrasound report from another hospital showed a mixed echoic mass in hepatic segments VII and VIII that measured about 4.3├Ś3.0 cm in diameter. About 10 years ago, he had taken renal biopsy due to proteinuria, however, the biopsy findings revealed no specific disease. He denied tobacco and alcohol use.
On physical examination, the patient was not icteric, but had right upper quadrant abdominal tenderness. Laboratory findings taken on admission were as follows: Hb 12.2 g/dl, WBC 5.8├Ś103/┬Ąl, platelets 164├Ś103/┬Ąl, urea nitrogen 34.0 mg/dl, creatinine 3.6 mg/dl, aspartate aminotransaminase 22 IU/l, alanine aminotransaminase 15 IU/l, total bilirubin 0.37 mg/dl, amylase 183 IU/l, lipase 140 IU/l, HBsAg negative, anti-HCV negative, rapid plasma reagin non-reactive, alpha-fetoprotein 1.7 ng/mg, PIVKA II 23 mAU/ml, CA19-9 574.8 U/ml. A 24-hour urinary protein excretion was 1,498 mg/day.
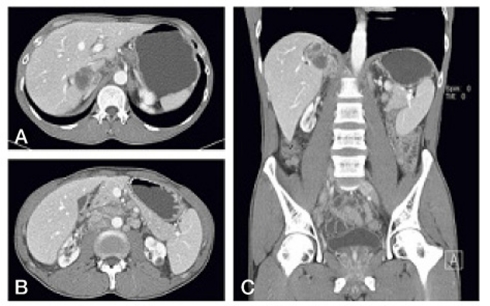
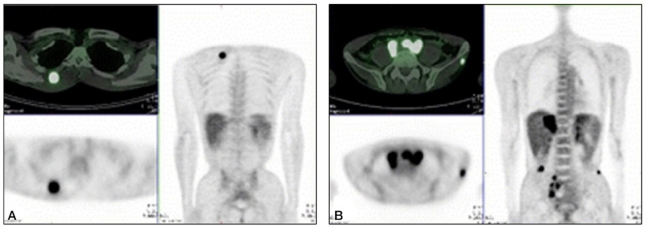
A contrast-enhanced CT of the liver showed a low-density mass in hepatic segments VII and VIII and enhanced lymph nodes, including the hepatoduodenal ligament and aortocaval and left para-aortic region, as well as a small amount of ascites in the early arterial phase (Fig. 1). A peripherally enhanced, low-density mass was revealed in the delayed phase. Intrahepatic cholangiocarcinoma was diagnosed based on the CT findings. He underwent 18F-FDG PET, which showed a malignant mass in the right hepatic lobe (p-SUV=11.2), a metastasis to the retroperitoneal and right common iliac lymphatic chain, and a distal paraesophageal area around the celiac axis. A paraspinal muscle metastasis in the right thoracic back (p-SUV=8.9) and left gluteus minimus muscle metastasis (p-SUV=4.5) were also revealed (Fig. 2).
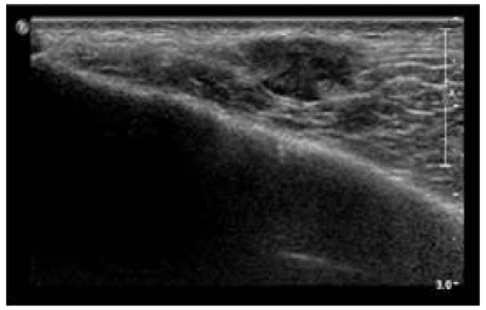
An ultrasound-guided percutaneous needle biopsy of the low echoic mass was performed at the left buttock area near the left iliac crest (Fig. 3). The pathologic findings revealed an adenocarcinoma that was positive for cytokeratin 7 and 19 but negative for cytokeratin 20 and CEA (Fig. 4).
The abdominal CT, 18F-FDG PET findings, and histopathologic features were compatible with intrahepatic cholangiocarcinoma with a distant skeletal muscle metastasis.
DISCUSSION
Biliary tract cancers are divided into cancers of the gall bladder, extrahepatic ducts, and the ampulla of Vater, and intrahepatic biliary tumors are classified as primary liver cancers.1 Intrahepatic cholangiocarcinoma is a malignant tumor arising from the epithelial cells of the intrahepatic bile ducts. Intrahepatic cholangiocarcinomas initially present as mass-forming lesions, and obstructive symptoms are rare.4 Fever, night sweats, and weight loss may occur in addition to right upper quadrant abdominal pain.4 Without clear reasons, the incidence of intrahepatic cholangiocarcinoma has been increasing over the past two decades in Europe, North America, Asia, Japan, and Australia.5 Some of this may be attributable to new diagnostic methods identifying biliary malignancies that have previously not been diagnosed.6
The major metastatic pattern for intrahepatic cholangiocarcinomas is early lymphatic spread. The hepatoduodenal ligament is the most common site for lymph node metastasis irrespective of tumor location. In the majority of cases, intrahepatic cholangiocarcinomas involve the lymph nodes of the hepatoduodenal ligament or those along the common hepatic artery.7,8 Cholangiocarcinomas have often metastasized to distant organs, and 18F-FDG PET is of value in discovering unsuspected distant metastases of cholangiocarcinoma.3,9 Other investigators showed that nine distant metastatic lesions were detected by 18F-FDG PET that were not detected using other imaging modalities.10 Because surgical resection is the only curative treatment of intrahepatic cholangiocarcinoma, accurate staging with 18F-FDG PET is useful for appropriate management.
Although skeletal muscle accounts for approximately 50% of the total body mass and receives a large volume of blood, distant skeletal muscle metastasis is very rare.11 The reason is not well elucidated. The effect of lactic acid on tumor cell production,12 the influence of variable and turbulent blood flow, ╬▓-adrenergic stimulation, tissue oxygen levels, and host immune response may provide protective mechanisms to skeletal muscle.13 Moreover, protease inhibitors in the muscle extracellular matrix may resist cancer cell invasion.14
Skeletal muscle metastasis is not generally explored until patients complain of symptoms such as localized pain or a palpable mass. Besides, intrahepatic cholangiocarcinomas are highly lethal, as most are locally advanced at presentation. This might be why distant skeletal muscle metastases from intrahepatic cholangiocarcinomas are rarely reported.
In our case, asymptomatic skeletal muscle metastasis was diagnosed by 18F-FDG PET during the initial examination in the patients with intrahepatic cholangiocarcinoma.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




