Chronic hepatitis B in pregnancy: unique challenges and opportunities
Article information
Abstract
Chronic hepatitis B (CHB) affects 350 million individuals worldwide. Perinatal transmission leads to high rates of chronic infection and complications, including cirrhosis and hepatocellular carcinoma. It is important to recognize and appropriately treat CHB in pregnancy, thereby reducing the risk of neonatal transmission and HBV-associated morbidity and mortality. Screening for CHB is recommended in all pregnant mothers as is universal vaccination of infants with hepatitis B virus (HBV) vaccine with or without hepatitis B immunoglobulin (HBIG). This has resulted in a lower incidence of HBsAg seropositivity and HCC in regions where universal infant vaccination has been endorsed. Mode of delivery and breastfeeding do not appear to affect HBV transmission rates based on available data. Overall, CHB does not increase perinatal maternal-fetal mortality. Administration of oral antiviral therapy during the third trimester to HBsAg-positive mothers with HBV DNA≥7 log IU/mL may be useful in preventing breakthrough infection. Treatment may be considered earlier in pregnancy for persistently active liver disease shown by high ALT, HBV DNA levels and/or significant hepatic fibrosis. Lamivudine, tenofovir and telbivudine are safe and effective and are the agents of choice in pregnancy. However, further clinical studies are necessary to elucidate the role of antiviral therapy in the pregnant HBV carrier.
INTRODUCTION
Chronic hepatitis B (CHB) in pregnancy is a prevalent and important problem with unique challenges. Over 50% of the world's 350 million carriers of CHB acquire the infection perinatally; in hepatitis B e antigen (HBeAg)-positive mothers, rates of transmission are as high as 90%.1 The majority (> 95%) of perinatally acquired infection results in CHB infection, due to induction of an immune tolerant state of variable duration. Worldwide, CHB remains a major health threat; each year approximately 600,000 individuals die of complications such as acute liver failure, cirrhosis, and hepatocellular carcinoma (HCC).2 Therefore, prevention of perinatal transmission remains an important target in the struggle for global eradication of hepatitis B virus (HBV) infection.
The prevalence of hepatitis B surface antigen (HBsAg)-positive pregnant individuals varies with geographic location and ethnicity. In the USA, HBsAg prevalence is 6% in Asian women, 1% in African-Americans, 0.6% in non-Hispanic whites and 0.1% in Hispanics.1 In endemic areas such as China and South East Asia, the prevalence may be as high as 10-20%.3 Due to recent immigration patterns in North America, country of birth as well as ethnicity are important risk factors for perinatal acquisition of HBV.
This review will focus on strategies aimed at decreasing maternal-fetal transmission of CHB. HBsAg screening, HBV vaccination, mode of delivery and breastfeeding, and oral antiviral prophylaxis will be discussed. Based on studies of antiviral agents in pregnancy, we propose an algorithm for the prevention of perinatal transmission of HBV.
SCREENING
Due to availability of a safe and effective vaccine against HBV, perinatal screening for HBV has become standard in perinatal care. Screening allows for identification of infants requiring immunoprophylaxis with HBV vaccine and hepatitis B immune globulin (HBIG), antiviral treatment of pregnant carriers if indicated, and counseling of sexual and household contacts.1
Universal screening as opposed to risk factor-based screening of all pregnant patients for hepatitis B is standard of care, since the latter results in missing as many as 50% HBsAg-positive individuals in some populations as shown by a study from Denmark.4 Therefore, the American Association for the Study of Liver Disease (AASLD) recommends that all pregnant women be screened for HBsAg during the first trimester, even if previously vaccinated or tested.5 Similarly, the US Preventive Services Task Force (USPSTF) recommends screening at the first prenatal visit.6 In practice, a positive HBsAg test must be communicated to the hospital where the patient intends to deliver, in order to allow for appropriate immunoprophylaxis. Furthermore, all HBsAg-positive pregnant carriers should ideally be referred for counseling and appropriate medical management.
VACCINATION
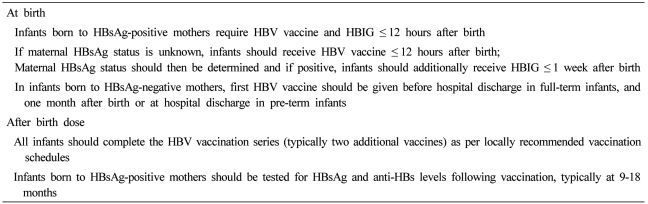
Since the development of the recombinant HBV vaccination in 1982, several health authorities, including the World Health Organization (WHO) recommend its use in infants born to mothers positive for HBsAg, in addition to other high-risk groups (Table 1). Globally, over 160 countries endorse universal infant vaccination, particularly in regions where HBV is endemic. WHO recommends the 1st dose of HBV vaccine administered within 24 hours of birth and 2 to 3 subsequent doses as part of routine immunization schedules. Hepatitis B immunoglobulin (HBIG) passive immunization in conjunction with HBV vaccination may also be administered to infants born to HBeAg-positive mothers. However, WHO acknowledges the limitations related to cost and supply of HBIG in certain endemic areas.2 The Centre for Disease Control (CDC) also advises one dose of HBV vaccine given shortly after birth with or without HBIG.7 The USPSTF recommends administering the first dose of HBV vaccine and HBIG within 12 hours of birth.8 Several studies have documented the benefits of such vaccination strategies in reducing HBsAg prevalence.9-14
Beasley et al reported results of a randomized blinded controlled trial of HBIG plus HBV vaccine for the prevention of perinatal transmission in 172 infants of HBeAg- positive mothers followed for up to 2 years after birth. Overall, there was a significant difference in persistent HBsAg positivity in 6% (9/156) infants who received immunoprophylaxis compared to 88% historical controls. There was no difference in efficacy between combined HBIG and HBV vaccination (94%), compared to HBIG alone (71%), or HBV vaccination alone (75%).9 A similar study by Wong et al. examined four vaccination schedules in 140 infants born to HBeAg-positive mothers. HBV vaccine was given at 0, 1, 2, and 6 months after birth. Persistence of HBsAg (≥ 6 months) was significantly lower in the treatment groups receiving vaccination plus 7 HBIG injections (2.9%), vaccination plus 1 HBIG injection (6.8%), and vaccination alone (21%), compared with placebo (73%, P<0.0001). Multiple HBIG injections did not appear to confer significant additional benefit.10 A recent systematic review and meta- analysis of 29 randomized controlled trials reported an overall reduction in HBsAg seroprevalence using vaccine alone (RR=0.28) or in combination with HBIG (RR=0.08) compared to placebo. However, only a small number of high quality trials were included in these reviews and most studies (62%) included only HBeAg-positive mothers.11
Despite the limitations of published trials, universal vaccination schedules have been widely accepted and implemented to decrease the risk of perinatal transmission, and ultimately, the global burden of disease (Table 2). Long-term follow-up studies from Taiwan, where vaccination has been in place for more than 20 years, have shown significant decreases in perinatal transmission rates and complications of CHB, including acute infection and HCC.12,13 Chang et al demonstrated that the incidence of HCC in Taiwan decreased in children aged 6-14 years from 0.70/100,000 (1981-1986) to 0.36/100,000 (1990-1994) (P<0.01).12 Although these studies suggest that universal infant vaccination is effective, not all infants respond to vaccine and chronic infection has been reported in 1-10% infants despite immunization due to breakthrough infection.11,14
ROLE OF ANTIVIRAL THERAPY
Risk factors for perinatal transmission of HBV include HBeAg-positive status and high HBV DNA, usually ≥108 copies/mL.1 The role of other factors such as viral genotype and mutation remain unclear. Strategies to prevent breakthrough infection despite vaccination include administration of HBIG during pregnancy and shortly after birth. More recently, nucleos(t)ide antiviral therapy such as lamivudine has been studied, analogous to the situation in human immunodeficiency virus (HIV)-infected pregnant women.
The role of antiviral therapy in pregnant HBV carriers has been addressed in a small number of studies. One small study included 8 HBeAg-positive mothers with high viral loads (≥1.2×109 geq/mL) who were treated with lamivudine 150 mg daily from 34 weeks gestational age to delivery compared to 24 infants born to untreated HBeAg-positive mothers who served as controls. All mothers received passive and active immunization. In the treatment group, only 1 (12.5%) infant remained HBsAg-positive at one year compared to 28% infants in the control group. HBV DNA declined by 1 log genome equivalents per mL (geq/mL) or more in 5 (63%) infants in the treated group (duration 6-40 days). No adverse events were reported in this small trial, suggesting that lamivudine was safe and effective in interrupting perinatal HBV transmission.15
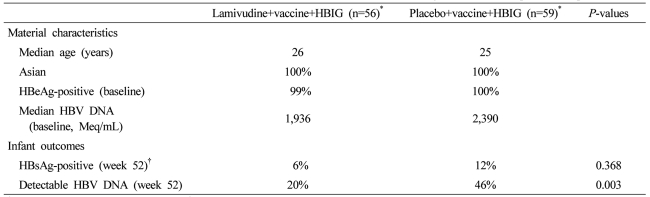
A large randomized placebo-controlled double-blind study of 155 HBeAg-positive women with HBV DNA≥1,000 Meq/ml but mainly normal ALT compared lamivudine 100 mg daily to placebo (Table 3). 141 infants received immunoprophylaxis, but only 115 who received both HBV vaccine and HBIG were included in the final analysis. Mothers randomized to lamivudine received treatment beginning at 32 weeks gestational age and ending at 4 weeks post partum. At 52 weeks post partum, 10/56 (18%) infants born to lamivudine-treated mothers remained HBsAg-positive compared to 23/59 (39%) infants in the control/standard of care group. HBV DNA remained detectable in 20% infants in the lamivudine group compared to 46% infants in the control group (P=0.003). No adverse events were reported. However, there were several important limitations in this study. An unexpectedly high rate of HBsAg seropositivity was seen in the standard of care group, suggesting that perhaps not all infants received immunoprophylaxis as per protocol. Additionally, infant loss to follow-up was significant. Taking this into account, reanalysis showed that there was only a trend but no significant difference in HBsAg seropositivity (6% lamivudine group vs. 12% control group).16
More data regarding the safety and efficacy of telbivudine in preventing perinatal transmission of HBV are emerging. Pan et al. compared outcomes in 53 HBeAg-positive mothers treated with telbivudine from second to third trimester until 4 weeks post-partum with 35 untreated control patients. In this study, baseline HBV DNA was > 6 log copies/mL and ALT >40 IU/mL but less than 10× upper limit of normal. All infants received active and passive immunization. At birth, 4% and 23% the newborns were HBsAg-positive in the telbivudine and control groups, respectively (P<0.001).17 Although these data are promising, more studies with longer follow-up are needed.
Safety data on antiviral therapy are primarily derived from clinical studies and the antiretroviral pregnancy registry, a prospective registry mainly for HIV-positive individuals. As of 2010, only 112 women with HBV mono-infection we included. The bulk of the clinical data has been reported with lamivudine and tenofovir, as experience with other antiviral agents such as entecavir, adefovir and telbivudine remains limited. Overall rates of birth defects on antiviral therapy are estimated to be 2.7% live births, which is comparable to prevalence in the general population.18 The FDA has classified entecavir, lamivudine, and adefovir as category C medications, whereas telbivudine and tenofovir are category B medications. Interferon has been classified as category X (Table 4).
The advantages of antiviral therapy during pregnancy include: potent antiviral suppression, relative safety and tolerability in pregnancy and, ultimately, a reduction in perinatal HBV transmission. Disadvantages include the risk of developing antiviral resistance in the mother depending on the antiviral agent used, contraindication to breastfeeding, and the risk of hepatitis flares upon discontinuation of therapy. In one study involving 31 HBeAg-positive and negative mothers, a postpartum flare defined as threefold increase in alanine amino transferase (ALT) occurred in 62% of lamivudine-treated mothers who discontinued therapy, compared with 42% of untreated mothers.19 Given limited long-term safety and efficacy data on antiviral therapy in pregnancy, the decision to initiate treatment should be made on a case-by-case basis.
In clinical practice, HBV DNA levels are measured during the second trimester in all pregnant HBsAg-positive mothers (Fig. 1). If HBV DNA is ≥7 log IU/mL then prophylactic treatment beginning in the early third trimester with lamivudine, tenofovir or telbivudine should be considered. Counseling should be provided regarding goals of treatment, treatment duration, potential side effects, overall safety in pregnancy for mother and fetus, monitoring on treatment and breastfeeding. If the mother decides to breast feed her infant, then all antiviral therapy must be discontinued at birth, in order to limit exposure to the infant. In addition, standard vaccination schedules are to be followed. Following cessation of therapy, mothers should be monitored with serial ALT and HBV DNA levels to detect asymptomatic treatment withdrawal flares, which may require subsequent treatment.
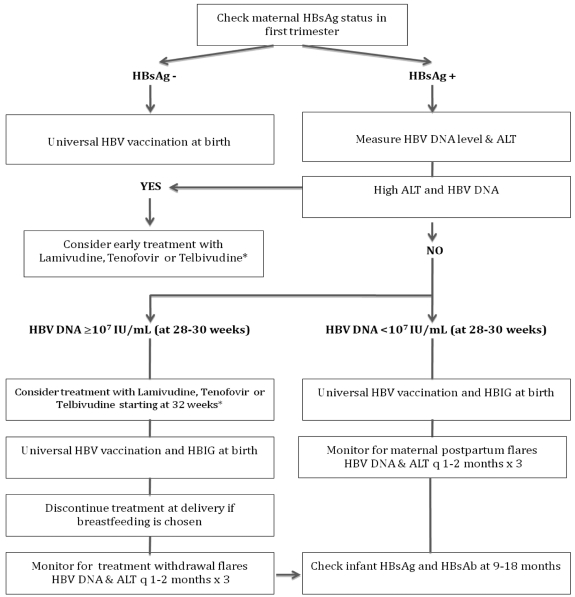
Algorithm for prevention of perinatal transmission of HBV infection.
*Appropriate counseling regarding treatment, treatment duration, potential side effects, monitoring on treatment, and cessation of therapy if breastfeeding is chosen.
HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; ALT, alanine aminotransferase; HBIG, hepatitis B immunoglobulin.
For HBsAg-positive carriers who develop active liver disease during pregnancy, standard treatment guidelines are applicable. The goal of treatment in this situation is to induce liver disease remission in the mother, in order to minimize the risk of preterm delivery. Persistent disease activity reflected by an elevated ALT, HBV DNA and/or presence of significant hepatic fibrosis (≥ F2 on Metavir) warrants early treatment, (ie. in the first trimester). Since long-term treatment may be required in this setting, treatment with tenofovir would be preferable due to overall safety and its very low risk of antiviral resistance.20-22
SPECIAL CONSIDERATIONS
Mode of delivery
Mode of delivery may also potentially affect the risk of perinatal HBV transmission, although different studies have yielded conflicting data. One study in China compared spontaneous vaginal delivery, vacuum extraction or forceps, and Caesarian section in terms of risk of HBV transmission. In total, 301 infants of HBsAg-positive mothers were included and all infants received HBIG at birth and hepatitis B vaccine shortly after birth. There was no difference in the rates of HBsAg positivity at birth between the 3 groups: 8.1%, 7.7%, and 9.7% of infants, respectively. Rates of chronic infection were not significantly different between the groups during follow-up.23 By contrast, a meta-analysis that included four randomized trials found that elective Caesarian section compared to vaginal delivery reduced mother-to-child HBV transmission (10.5% vs. 28%). Although the results were statistically significant, included studies had methodological flaws.24 Therefore, most obstetric guidelines do not endorse routine use of Caesarian section to prevent perinatal transmission of HBV.
Breast feeding
Transmission of HBV via breast milk is often another concern facing HBsAg-positive mothers. Early studies reported HBsAg, HBeAg and HBV DNA detection in colostrum. Higher HBsAg and HBeAg titres were found in mothers with high serum HBV DNA, suggesting that breast milk may be an important vehicle for transmission.25 However, in a population study of 369 vaccinated infants born to carrier mothers, HBsAg prevalence in breast-fed infants was 0/101 (0%) compared to 9/268 (3%) formula-fed infants.26 Although this difference was not significant, it suggested that breast milk may have antiviral properties due to immunoglobulins and other proteins such as lactoferrin found in breast milk, which may explain the lower prevalence of HBsAg in breast-fed infants.27 Previous studies had also shown no increased risk of transmission with breastfeeding.28 In view of the multiple benefits of breastfeeding, WHO recommends breastfeeding for infants of HBsAg-positive mothers even in endemic areas where HBV vaccination may not be readily available.29 However, the issue remains controversial and other organizations such as the American Academy of Pediatrics suggest that breast feeding not be withheld, provided that infants receive HBV vaccine and HBIG.30
Nucleos(t)-ide therapy during breastfeeding is not recommended. Human data on lamivudine and other antiviral agents during breastfeeding in HBV mono-infection are not available.27 One study that compared infant hematologic and hepatic toxicity of maternal highly active antiretroviral therapy for HIV infection via breast milk found no significant difference between breastfed and formula-fed infants.31 However, until more clinical data on the safety of tenofovir and other antiviral agents during breastfeeding are available, antiviral treatment during breastfeeding in HBV patients remains contraindicated.
MATERNAL AND FETAL OUTCOMES
Even though CHB may be associated with significant mortality in non-pregnant chronic carriers, HBV infection during pregnancy does not appear to increase maternal or fetal mortality and morbidity. One large study that compared 824 HBsAg-positive mothers to 6281 HBsAg-negative but otherwise similar control patients found no difference in preterm delivery, birth weight, neonatal jaundice, congenital anomalies, as well as perinatal mortality.32 However, a more recent case-control study compared outcomes of 253 HBsAg-positive pregnant carriers to 253 matched controls. On multivariate analysis, HBsAg carriers had an increased risk of gestational diabetes mellitus, antepartum hemorrhage, and threatened preterm labour. This may be explained by active liver disease related to HBV infection during pregnancy in a proportion of patients included, which may have predisposed to the development of obstetric complications.33 However, mortality was not significantly different between the groups and further studies are needed to determine the relationship between CHB and maternal and fetal complications.
CONCLUSION
CHB in pregnancy presents a unique challenge, but also an important opportunity to interrupt perinatal transmission of HBV. Since maternal-fetal transmission is the major route of acquisition of HBV worldwide, strategies to eradicate HBV or to reduce global burden of disease must target this critical step in HBV disease propagation. To this end, population-based screening of all pregnant women for HBV and universal infant vaccination are required. Standard of care for the HBsAg-positive mother includes HBV vaccination and HBIG in most centers in North America. Chronic HBV infection does not seem to increase risk of maternalfetal morbidity and mortality. Additionally, standard vaginal delivery and breastfeeding do not appear to increase the risk of HBV transmission. Antiviral therapy using lamivudine, tenofovir or telbivudine should be reserved for those who have active liver disease during pregnancy and for those with very high viral load HBV DNA>7 log IU/mL in the third trimester, after an informed discussion with the pregnant HBV carrier. However, further large clinical trials are required to determine the role of antiviral prophylaxis in pregnancy. In summary, early recognition of the pregnant HBV carrier followed by treatment with safe antiviral agents, if indicated, and immunoprophylaxis will reduce perinatal HBV infection and its complications, while minimizing risk to the fetus/infant.
Abbreviations
AASLD
American Association for the Study of Liver Disease
ALT
alanine aminotransferase
CDC
Centre for Disease Control
CHB
chronic hepatitis B
FDA
Food and Drug Administration
HAART
highly active antiretroviral treatment
HbeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBIG
hepatitis B immune globulin
HBV
hepatitis B virus
HIV
human immunodeficiency virus
HCC
hepatocellular carcinoma
USPSTF
United States Preventive Services Task Force
WHO
World Health Organization