| Korean J Hepatol > Volume 17(1); 2011 > Article |
ABSTRACT
Background/Aims
Methods
Results
Abbreviations
REFERENCES
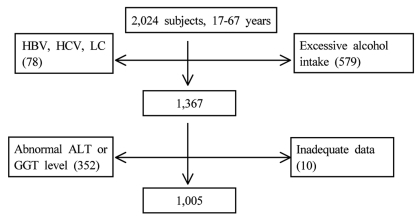
Figure┬Ā1
Table┬Ā1
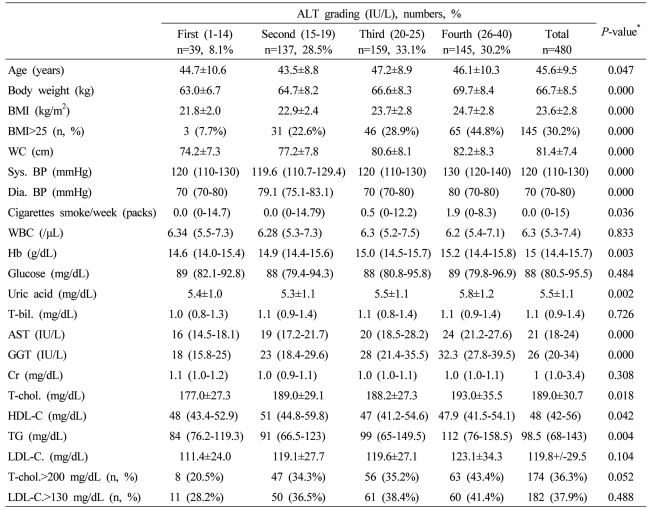
Data are expressed as means┬▒SD, medians (interquartile ranges) for skewed variables, or proportions for categorical variables. Differences were assessed using the Kruskal-Wallis H test for continuous variables or the chi-square tests for categorical variables.
*Tested by the Spearman's rank correlation analysis.
WC, waist circumference; Sys. BP, systolic blood pressure; Dia. BP, diastolic blood pressure; T-bil., total bilirubin; AST, aspartate aminotransferese; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; Cr, creatinine; T-chol., total cholesterol; LDL-C., LDL cholesterol; TG, triglyceride; HDL-C., HDL-cholesterol.
Table┬Ā2

Data are expressed as means┬▒SD, medians (interquartile ranges) for skewed variables, or proportions for categorical variables. Differences were assessed using the Kruskal-Wallis H test for continuous variables or the chi-square tests for categorical variables.
*Tested by the Spearman's rank correlation analysis.
WC, waist circumference; Sys. BP, systolic blood pressure; Dia. BP, diastolic blood pressure; T-bil., total bilirubin; AST, aspartate aminotransferese; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; Cr, creatinine; T-chol., total cholesterol; LDL-C., LDL cholesterol; TG, triglyceride; HDL-C., HDL-cholesterol.
Table┬Ā3
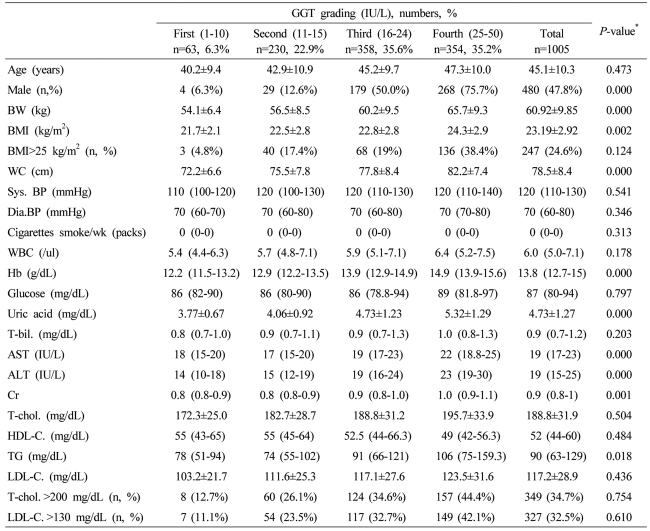
Data are expressed as means┬▒SD, medians (interquartile ranges) for skewed variables, or proportions for categorical variables. Differences were assessed using the Kruskal-Wallis H test for continuous variables or the chi-square tests for categorical variables.
*Tested by the Spearman's rank correlation analysis.
WC, waist circumference; Sys. BP, systolic blood pressure; Dia. BP, diastolic blood pressure; T-bil., total bilirubin; AST, aspartate aminotransferese; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; Cr, creatinine; T-chol., total cholesterol; LDL-C., LDL cholesterol; TG, triglyceride; HDL-C., HDL-cholesterol.
Table┬Ā4
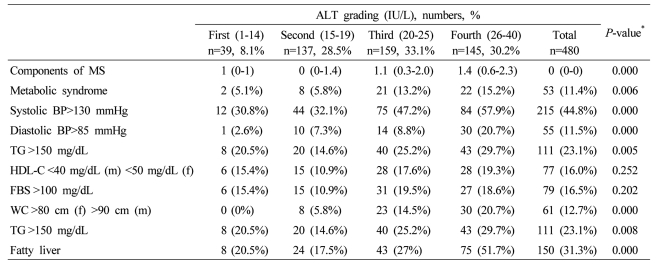
Data are expressed as medians (interquartile ranges) for skewed variables, or proportions for categorical variables. Differences were assessed using the Kruskal-Wallis H test for continuous variables or the chi-square tests for categorical variables.
*Tested by the Spearman's rank correlation analysis.
MS, metabolic syndrome; WC, waist circumference; TG, triglyceride; FBS, fasting blood glucose; HDL-C, HDL cholesterol.
Table┬Ā5
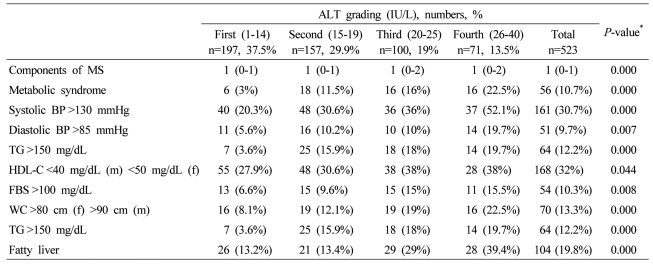
Data are expressed as medians (interquartile ranges) for skewed variables, or proportions for categorical variables. Differences were assessed using the Kruskal-Wallis H test for continuous variables or the chi-square tests for categorical variables.
*Tested by the Spearman's rank correlation analysis.
MS, metabolic syndrome; WC, waist circumference; TG, triglyceride; FBS, fasting blood glucose; HDL-C, HDL cholesterol.
Table┬Ā6
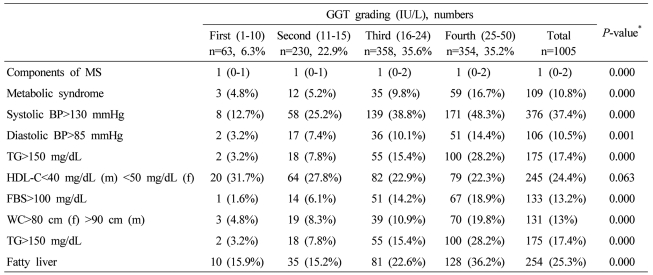
Data are expressed as medians (interquartile ranges) for skewed variables, or proportions for categorical variables. Differences were assessed using the Kruskal-Wallis H test for continuous variables or the chi-square tests for categorical variables.
*Tested by the Spearman's rank correlation analysis.
MS, metabolic syndrome; WC, waist circumference; TG, triglyceride; FBS, fasting blood glucose; HDL-C, HDL cholesterol.
Table┬Ā7
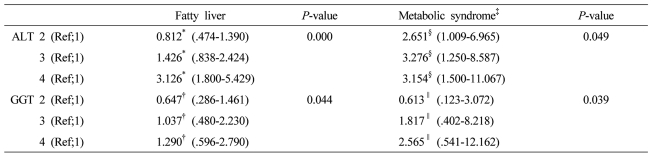
The factors (ALT grading and GGT grading) correlated with fatty liver or metabolic syndrome were evaluated using the multiple logistic regression analysis after adjustment for clinical and biochemical parameters. The several variables with P less than .1 in univariate analysis were included.
*Adjusted for obesity, systolic blood pressure >130 mmHg, diastolic blood pressure >85 mmHg, metabolic syndrome, fasting blood sugar, GGT, ŌĆĀAdjusted for obesity, systolic blood pressure >130 mmHg, diastolic blood pressure >85 mmHg, metabolic syndrome, fasting blood sugar, ALT, ┬¦Adjusted for age, sex, systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg, HDL cholesterol, fatty liver, body weight, GGT, ŌłźAdjusted for age, sex, systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg, HDL cholesterol, fatty liver, body weight, ALT ALT grading (first 1-14 IU/L, second 15-19 IU/L, third 20-25 IU/L, and fourth 26-40 IU/L) GGT grading (first 1-10 IU/L, second 11-15 IU/L, third 16-24 IU/L, and fourth 25-50 IU/L).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




