Epidemiology and prevention of hepatitis B virus infection
Article information
Abstract
Hepatitis B virus (HBV) infection has been a major global cause of morbidity and mortality. The recognition of the problem led to a worldwide effort to reduce transmission of HBV through routine infant vaccination. HBV infection is the most common cause of chronic liver diseases and hepatocellular carcinoma in Korea. After hepatitis B vaccine era, seroprevalence of hepatits B surface antigen is decreasing, particularly in children. Hepatitis B vaccine is remarkably safe and shows high immunogenicity. Universal childhood immunization with three doses of hepatitis B vaccine in the first year of life is a highly effective method for prevention and control of hepatitis B.
INTRODUCTION
Hepatitis B is an infectious disease, associated with an estimated 350 million chronically infected patients.1 Perinatal transmission is an important cause of hepatitis B virus (HBV) transmission, account for 35% to 50% of HBV carriers.2 Korea is an endemic area of HBV infection, therefore, hepatitis B is an important public health problem. This review describes an overview of HBV infection, epidemiology, immunization, and prevention.
HEPATITIS B VIRUS
HBV is a 42-nm DNA virus in the family Hepadnaviridae. The virus has a partially double-stranded DNA with core antigen surrounded by a shell containing surface antigen (HBsAg).3 HBV contains numerous antigenic components, including HBsAg, HBcAg, and hepatitis B e antigen (HBeAg). The HBV genome has 3200 nucleotides, and it efficiently uses its genetic information to encode four groups of proteins and their regulatory elements by shifting the reading frames over the same genetic material.4 The immune response to HBsAg provides the immunity against HBV. Antibodies to HBcAg indicate infection, IgM anti-HBc indicates recent infection and usually disappears within six months, while IgG anti-HBc persists for life and indicates past infection. Antibody to HBsAg (anti-HBs) appears after clearance of HBsAg or after immunization. The presence of HBsAg for more than six months is defined as chronic HBV infection.
MODE OF TRANSMISSION
HBV is transmitted parenterally by contaminated blood or other body fluids through blood vessels, skin or mucous membranes. The virus can be detected in all human body fluids, the virus concentration in the fluids is highest in the blood or serous exudates, and it is relatively low in saliva, semen, vaginal fluids.5 Thus, there is almost no risk of transmission in the course of daily life, and the infection through fecal-oral transmission does not occur.
The main transmission routes include perinatal infection, the skin and mucous membrane infections caused by contaminated blood or body fluids (blood transfusion, use of contaminated syringes, hemodialysis, invasive tests or surgery), and sexual contacts. In Korea, the most important route is perinatal transmission in patients with chronic hepatitis B.6
Perinatal infection occurs frequently if the mother with HBV is HBeAg-positive and has a high level of serum HBV-DNA. If infants born to HBsAg-positive mother do not receive perinatal hepatitis B vaccine and immunoglobulin prophylaxis, infection rates in infants born to HBsAg positive and negative mother are 70 to 90% and 10 to 20%, respectively, of which 90% will progress to chronic infection.7,8 When given with vaccine and immunoglobulin within 24 hours after birth, 90% of infants show the preventive effect.9 Although a very small amount of HBV is detected in breast milk, there is no evidence that hepatitis B is transmitted through breast milk.
In injection drug abuser, using the same syringe among injection drug users at the same time is the main transmission route of hepatitis B infection.10 In addition, tattooing, ear piercing, acupuncture, dialysis, and even using a syringe can be the source of infection.11
In adolescents and adults in the countries with low and middle prevalence rates of hepatitis B, sexual contact is the major cause of HBV infection.12 Recently, as the prevalence of HBV infection is decreasing in young adult age group in Korea, the sexual contact has become the main transmission route.
CLINICAL FEATURES
Hepatitis B shows variable clinical manifestations ranging from asymptomatic HBV carriers to fulminant liver failure, and it becomes chronic, often progresses to chronic hepatitis, cirrhosis, and hepatocellular carcinoma.13 These clinical manifestations may vary depending on their age at infection, showing symptoms in less than 10% of children under 5 years old, while causing symptoms of the infection in 30 to 50% in adults.14 The risk of chronic HBV infection varies inversely with age; 80 to 90% of neonatal infections, 30 to 60% of infants, 5% or less of adults.15 The majority of cases of vertical transmission from mother progresses to chronic carriers.
The incubation period for the onset of acute hepatitis B is 3 to 4 months, showing prodromal symptoms such as fatigue, fever and anorexia, which are similar to other viral hepatitis. Serum-sickness syndromes such as joint pain and rash were observed in about 10% of patients.16 Jaundice occurs at 1 or 2 weeks after the onset of symptoms, and clinical symptoms are improved in the majority of cases within 3 months. Fulminant hepatitis, accompanied by hepatic encephalopathy is complicated in 0.5% of cases in adult patients.17 In most cases of acute hepatitis B, HbsAg disappears within 6 months and liver function is recovered. When the virus persists longer than 6 months, it becomes a chronic state. The natural history of chronic hepatitis B is determined by the immune response and viral replication. The initial phase of perinatally acquired infections is characterized by high levels of virus replication with no evidence of active liver disease with normal transaminase levels due to immune tolerance.18 The second phase of perinatally acquired chronic infection, immune clearance phase, is characterized by high levels of virus replication with active liver disease. Fibrosis of the liver can develop during this phase, which can lead to cirrhosis. During the immune clearance phase, seroconvesion of HBeAg and decrease in HBV DNA level may occur, hereafter staying as 'inactive HBV carriers' with low viral load and normal liver function.19 Some patients show repeated increase in virus levels and necroinflammation, progressing to cirrhosis or liver cancer over a long period of time.20 The clinical course of hepatitis B is determined by the interaction of viral replication status and host immune response. Otherwise, it can be worsened by the factors such as alcohol and coinfection with other viruses. The risk of progression to decompensated liver disease or the incidence of liver cancer increases with high viral replication status and the risk is higher in patients with cirrhosis than hepatitis.21 It has been known that the risk of liver cancer is increased further in patients with older age, family history of liver cancer or cirrhosis, alcohol and the coinfection of hepatitis C virus.22
EPIDEMIOLOGY
Hepatitis B is the most common and important cause of cirrhosis and hepatocellular carcinoma in Korea, accounting for approximately 70% of the causes.23
HBV is infected by parenteral route through blood or body fluids. In the past, blood transfusions were the mainstream infections, but now there are almost no infections due to the introduction of screening tests for HBV in blood donors and vaccinations. In the areas with high prevalence rate of HBV as in Korea, perinatal transmission from mother to child is the major route of infection.24 In addition, infections through sexual transmission or needle sharing are possible transmission route in adults.
Until the 1980s before the introduction of hepatitis B vaccine in Korea, the prevalence rate of HBV was almost 8% of the general population.25 Perinatal infection rate and intrafamily infections were very high in the infant and childhood.26 Hepatitis B vaccination was introducted in Korea since 1985, and immunization for all neonates and the active and passive immunization for newborns of HBsAg positive mothers have been performed since 1991. After vaccine era, the prevalenc of HBV infection in Seoul metropolitan area was markedly reduced to 0.6% in children under 10 years old, to about 1.6% in the teens, and also it was reduced to 3% in the early 20s.27 However, the prevalenc rate in 20s and over showed 7.6% in men and 3.4% in women, respectively, indicating no significant differences to the prevalence rates in the past. Perinatal infection in HBV carriers was also as high as 54.7% in age 20 and over, while it was reduced to 7.4% in age less than 10.28 Hepatitis B vaccine has been included in a National Immunization Program, and since 95% of infants are vaccinated, the neonatal hepatitis B prevention in Korea is being implemented effectively.29 Recently, the HBsAg positive rate for those under school age is very low at less than 1%.30 Twenty years after the introduction of the vaccine, the prevalence of HBV infection is decreasing in adolescence and young adults as well as in the ages of infants and children. A nationwide sero-epidemiological survey is needed to provide immunization strategies in adult population.
The occurrence of acute hepatitis B also decreased, and in recent years, it became rarely observed in age groups under 20.31 The phenomenon of the decrease in young patients with acute hepatitis B is similar to other countries such as Taiwan or the United States, and it is consistent with the time for introduction of hepatitis B vaccination.32-34 The reduced incidence of acute hepatitis B in youth ultimately implies the reduced incidence of acute hepatitis B infection for the society or country as a whole. On the other hand, the incidence of acute hepatitis B in the age group over 30 years is relatively high compared to the younger age groups, which might be due to many unvaccinated people.31
EFFICACY OF HEPATITIS B VACCINE
The ultimate goal of hepatitis B vaccination is to reduce the prevalence of chronic hepatitis B carriers, as well as to prevent the occurrence of acute hepatitis B. Vaccination is the most effective method for the decrease in the prevalence of HBV infection.
Hepatitis B vaccines have been evaluated in clinical trials to determine protective level of serum anti-HBs. Persons who respond to HBV vaccine with titers of anti-HBs 10 mIU/mL or greater are protected against acute hepatitis B and chronic infection.35 Antibody production rate is higher in the younger age at vaccination and lower for those older than 40 years of age. Thus, antibody test after vaccination is not required for healthy people, however, it should be tested after vaccination in hemodialysis patients, HIV positive people, those at occupational risk of infection such as workers in operation rooms and intensive care unit, babies born to HBsAg-positive mothers, and those with family history of HBV carriers. Since the antibody titer is at maximum around on 1 to 3 months after the completion of vaccine series, the tests for anti-HBs are performed at this time.
The antibody response to hepatitis B vaccine is 90% in healthy adults and 95% of infants, children, and adolescents.36 As for vaccine efficacy, complete protection has been achieved among immunocompetent persons who developed anti-HBs titers of 10 mIU/mL or greater after completion of vaccination.35,37
Long-term follow-up studies of newborn vaccination demonstrated that antibodies become negative in 15-50% among the vaccine responders within 5 to 10 years.33,38,39 In adults, antibodies become negative in 7-50% within 5 years, and 30-60% within 10 years.39,40 In the cohort study for 20 years after infant vaccination, booster vaccination in cases of waning antibody produced antibody response rate in excess of 80%, and HBV infection did not occur during the period of the cohort.41 The persistent protection against clinically significant hepatitis B despite of declining antibody titers is an anamnestic immune response after HBV exposure.42 This indicates that the immune system would be able to respond rapidly to HBV infection. At present, routine booster doses of hepatitis B vaccine or periodic serologic test to monitor antibody titiers for immune-competent persons who have responded to vaccination is not recommended.43 However, additional vaccinations are required for immunocompromised patients such as patients with chronic renal failure if antibodies become negative in antibody testing every year after vaccination.44 Persons who have anti-HBs titer after completion of primary vaccine series less than 10 mlU/mL is called 'vaccine nonresponders'. Several factors has been associated with nonresponse to HBV vaccination. These include inappropriate vaccine storage conditions, vaccination in buttocks, old age, obesity, hemodialysis, chronic liver disease, and state of immunosuppression, and presence of DR3, DR7 DQ2 and absence of A2 alleles.45,46 Approximately 5-10% of vaccinated person do not achieve detectable antibody response (nonresponders).47 Further vaccination of the nonresponders administered in the deltoid muscle produces adequate antibody response in 15% to 25% of them after one additional dose and in 30% to 50% after three additional doses.48
There are several approaches to improve the antibody response to vaccine. Obesity can be a major cause of lowering the rate of antibody production by the vaccine, recently it has been reported that antibody titers were raised by using long needles.49 The research for developing new vaccines to improve immunity of the vaccine also has been actively conducted. The vaccines including the Pre-S antigen produced by recombinant DNA methods using CHO cells, or Fendrix® containing MPL (3-O-desacyl-4-monophosphoryl lipid A) as an additive to conventional vaccine components can increase the reaction of antibody generation by the vaccine and continue the immune response.50 In addition, the method to reduce the number of vaccinations to two times in order to increase vaccination rates and compliance of vaccination, various forms of combination vaccines containing HBV components have been developed and currently the combination vaccines with HAV, DTP or Hib have been used.51,52 Hepatitis B vaccine has no significant increase in adverse reactions and no interference in antiobody response of other immunizations such as DPT, polio, measles, Hib, or hepatitis A.53,54 In addition, even when vaccinated with other types of hepatitis B vaccination after a hepatitis B vaccines, there is no difference in vaccine effectiveness. In recent years, the vaccination methods through skin or mucous membranes other than traditional intramuscular injection have been introduced by developing new antigen delivery systems.55
Hepatitis B vaccination decreases HBV infection and ultimately reduce the incidence of chronic liver disease or liver cancer. It was reported that incidence of hepatocellular carcinoma in children decresed more than 50% after universal infant and childhood vaccination.56 In Korea, neonatal vaccination has been conducted and HBsAg positive rate in young adults have recently been greatly reduced, showing only about 3% of HBV infection rate, and it has been decreased less than 1% in children.27 The incidence of acute hepatitis B has been also reduced, therefore, the incidence of chronic liver diseases or hepatocellular carcinoma by HBV infection could be expected to decrease in the future.
PREVENTION
Preventive measures of HBV infection include the avoidance of high-risk behaviors, the prevention of exposure to blood and body fluids, the screening of women in pregnancy for HBV, active immunization or passive immunization before or after exposure. There are active immunization method using vaccines and passive immunization with HBIG.
Active immunization
Types of vaccines
Plasma vaccines isolated from HBV carriers have been used since 1980s. The plasma vaccines were developed in the early days of vaccines in Korea, and plasma vaccines was used from 1983.57 Plasma vaccines produce good immunogenecity and they are effective and safe. However, they are rarely used in recent years due to the shortage of plasma of HBsAg carriers, the concerns for the safety of blood product, and the development of recombinant DNA technology. Recombinant DNA vaccines are produced by introducing HBsAg into yeast or mammalian cells (CHO; Chinese Hamster Ovary cell).58 Antibody conversion rates and geometric means of the generated antibody titers by recombinant vaccines are similar to the plasma vaccines.36
Combination vaccines containing a hepatitis B vaccine component are developed. These include DTP-HepB vaccine, DTP-Hib-HepB vaccine, DTP-Hib-inactivated poliovirus vaccine-HepB vaccine, Hib-HepB vaccine, and hepatitis A-HepB vaccine.51,52
Vaccine dose and method
Hepatitis B vaccine includes 20 µg of surface antigen for adults, and 10 µg for children. The vaccine should be given by intramuscular injection in anterolateral aspect of thigh of infants and children less than 2 years of age, and in the deltoid muscle of older children, adolescents, and adults. Injection in the buttocks is not recommended because of the possibility of subcutaneous inoculation into deep fat tissue, which is associated with decreased protective antibody levels.59 There is no interference effect of simultaneous vaccination with any other vaccines, and in this case, injection site should be in other parts or at least 1.5 cm apart from the hepatitis B vaccine inoculation site.
Hepatitis B vaccine is generally administered in three doses, with the second dose given one month after the first dose and the third dose given six months after the first dose. Vaccines from the other manufactures can be used interchangeably. If the series is interrupted after the first dosem the second dose should be given as soon as possible and the second and third doses should be separated by an interval of at least 2 months. If the third dose is delayed, it should be given as soon as possible. It is not necessary to restart the vaccine series when there has been prolonged interval between the doses.60,61
Vaccination shall be given at 0, 1, and 6-month intervals. A second vaccination shall be given at one month after the first vaccination, and in case of missing the vaccination schedule, without having to start again, it shall be given right away, and a third vaccination shall be given at the interval of two months or more after second vaccination.
Storage and safety of vaccines
Most hepatitis B vaccines should be kept in 2-8℃ storage temperatures, and the efficacy is maintained at least 4 years. Vaccine efficacy is maintained at room temperature and can be stored for up to one year. Vaccine should not be frozen because the freezing causes the HbsAg protein to dissociate from the alum adjuvant and lose immungenicity.62
Serologic testing
Serologic testing is not indicated before routine vaccination of infants or children. Screening test in adults is considered in the endemic area of HBV infection or in the groups with a high risk of HBV infection. Serologic test following vaccination in healthy adults is not recommended. However, antibody tests should be considered in health care workers who are at the high risk of infection or in the cases of hemodialysis patients, HIV-infected patients, and spouses of hepatitis B patients. Post-vaccination testing should be performed 1-2 months after completion of the vaccine series.
Vaccine non-responder
Anti-HBs response less than 10 mIU/mL after completion of the first vaccine series is called 'nonresponders'. Re-vaccination is recommended in the nonresponders. Administration of a single dose of vaccine, followed by measurement of anti-HBs antibody response 1-3 months later to differentiate anamnestic response. For persons who are seronegative after the booster dose, a second vaccine series of two additional doses, which result in seroconversion in 50 to 60% of recipients.48 Generally, additional vaccination is not recommended to the complete nonresponders who do not achieve the appropriate antibody level even after a total of 6 times of vaccinations. HBIG should be administered when they are exposed to HBV.
Adverse reactions
Hepatitis B vaccine is safe and mild side effects appear within 24 hours, showing fever in 1-6%, injection site pain in 3-29%, redness and mild swelling in 3%, and headache in 3%.63,64 Arthritis, multiple sclerosis, diabetes, Guillain-Barre syndromes have no direction association to the hepatitis B vaccination.65 Hypersensitivity to vaccine components, such as yeast, can cause anaphylaxis.66
Indications
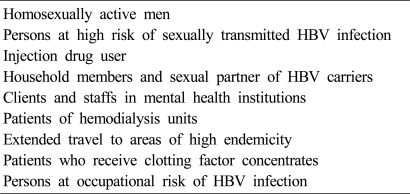
Comprehensive HBV immunization strategies prevent chronic HBV infection acquired in all age groups. In the past, vaccination to all newborns was performed mainly in the hepatitis B prevalent areas, but since 1997, hepatitis B vaccinations to newborns are being performed worldwide regardless of the prevalence of hepatitis B. Currently, vaccinations to all newborns are being performed and even in adults, catch-up vaccination is recommended in case of unvaccinated person without anti-HBs.43,54 It should be particularly recommended to high-risk groups of hepatitis B (Table 1).67
Contraindications
The vaccination is contraindicated in those who have a history of hypersensitivity to vaccine components or who have experienced severe side effects after the hepatitis B vaccination previously. There is limited data on the safety of hepatitis B vaccine for pregnant woman, however, there is no apparent adverse effect to fetus. Therefore, pregnancy or breast feeding are not a contraindication to vaccination.68 The vaccine can be given to immunocompromised patients, but the antibody response to the vaccination is lower than in healthy people.69
Passive immunization
Hepatitis B vaccination not only prevents hepatitis B but also can attenuate the severity of illness or inhibit the progress toward chronic phase even if they have already been infected and in the incubation period during vaccination. HBIG is recommended for postexposure prophylaxis in the setting of perinatal exposure, percutaneous or mucous membrane exposure to HBV, and sexual exposure to an HBV carrier. It is also used to reduce the risk of recurrent HBV infection after liver transplantation.70 HBIG is prepared from human plasma of those who contain a high titer of anti-HBs. HBIG is effective in preventing clinical hepatitis B or chronic infection, if used shortly after exposure to HBV.71
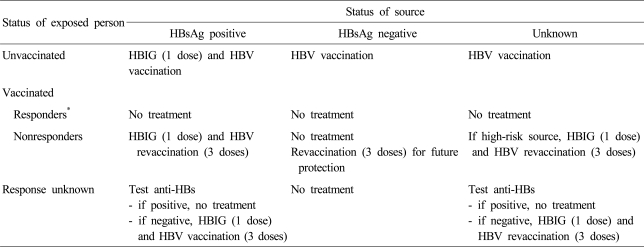
One of the major uses of HBIG is as an immuonprophylaxis in preventing perinatal HBV transmission. It has been reported that immunoprophylaxis with both HBIG and hepatitis B vaccine could increase the efficacy of preventing perinatal HBV transmission to 85% to 95% and provide long-term protection.72,73 HBIG in indicated for postexposure prophylaxis after needle stick injury or percutaneous exposure. In cases of the percutaneous and mucosal exposure to blood or body fluids, such as HBsAg-positive needle stick, the prophylactic procedures vary depending on the vaccination status of the exposed person and the status of antibody. The persons who have anti-HBs after vaccination do not need the vaccine or HBIG. In unvaccinated cases, HBIG 0.06 mL/kg, up to 5 mL is given by intramuscular injection. and the first dose of vaccine should be given within 24 hours after exposure (Table 2). In nonresponders, restart the vaccination after the injection of HBIG immediately. In nonresponders who did not complete re-vaccination after the primary vaccination, it is better to start again after 1 time of HBIG injection. In nonresponders even after 3 times of re-vaccination, it is preferred to perform 2 times of HBIG injection.
General measures
HBV is mainly transmitted through perinatal infection, sexual contact, blood, and contaminated needles from drug abusers. It can be transmitted through the direct contacts or skin wounds between patients and surgeons or dentists, or indirectly through hemodialysis, razors, toothbrushes, and acupuncture therapy.32 After exposure to HBV contaminated blood or body fluids, HBV contaminated blood should be removed and disinfect the contaminated instruments. In addition, education on the transmission routes to the public and the medical workers are needed for the prevention of HBV infection.
CONCLUSIONS
Hepatitis B immunization has made a hepatitis B a vaccine preventable infectious disease. The hepatitis B vaccine has been associated with decrease in the incidence of HBV infection, and ultimately reduced chronic liver diseases and hepatocellular carcinoma related to HBV infection. Universal vaccination program of infants and children has been successfully control the HBV infection in endemic areas including Korea. We also should pay attention to the prevention of perinatal transmission and HBV infection in unvaccinated adults.
Abbreviations
HBV
hepatitis B virus
HBsAg
hepatitis B surface antigen
HBeAg
hepatitis e antigen
Anti-HBs
antibody to HBsAg
HBIG
hepatitis B immune globulin