Regression of esophageal varices during entecavir treatment in patients with hepatitis-B-virus-related liver cirrhosis
Article information
Abstract
Recent studies suggest that liver cirrhosis is reversible after administering oral nucleos(t)ide analogue therapy to patients with hepatitis B virus (HBV) infection. However, few studies have addressed whether esophageal varices can regress after such therapy. We report a case of complete regression of esophageal varices during entecavir therapy in patients with HBV-related liver cirrhosis, suggesting that complications of liver cirrhosis such as esophageal varices can regress after the long-term suppression of HBV replication.
INTRODUCTION
Hepatitis B virus (HBV) infection is a worldwide public health problem. It is estimated that there are more than 350 million HBV carriers in the world [1]. HBV is the most common cause of chronic liver disease including liver cirrhosis and hepatocellular carcinoma [1]. Recently, potent oral nucleos(t)ide analogues (NA), including entecavir and tenofovir, have been widely used in the treatment of chronic hepatitis B (CHB) [2-5]. The potent antiviral treatment in patients with CHB can suppress the viral replication and prevent progression to cirrhosis, hepatic failure and development of hepatocellular carcinoma [6-12]. Furthermore, recent studies demonstrated that regression of liver cirrhosis could be achieved in 74-86% of patients who received NA therapy over 5 years [8,13]. However, there is little data showing that antiviral therapy can regress esophageal varices in patients with HBV-related liver cirrhosis. Herein, we report a patient with HBV-related liver cirrhosis, whose esophageal varices regressed during entecavir therapy.
CASE REPORT
A 48-year-old man visited outpatient clinic with abdominal discomfort. He was a hepatitis B surface antigen (HBsAg) carrier for long time. The physical examination showed shifting dullness and pitting edema. Laboratory findings were as follows: white blood cell count of 5,100/µL (N:4,000-10,000), hemoglobin of 15.3 g/dL (N: 13-17), platelet count of 1.79×105/µL (N:150,000-450,000), creatinine of 1.2 mg/dL (0.9-1.3), aspartate aminotransferase level of 442 IU/L (N:8-38), alanine aminotransferase (ALT) level of 414 IU/L (N:4-44), total bilirubin of 1.7 mg/dL (N:0.2-1.2), albumin of 3.3 g/dL (N:3.8-5.3), and prothrombin time of 1.1 international normalization ratio (INR) (N:0.88-1.2). HBsAg and hepatitis B e antigen (HBeAg) were positive. Anti-HBs and anti-HCV were negative. Serum level of HBV DNA was 3.87×105 IU/mL. Ultrasonography showed coarse echogenicity in liver parenchyma, nodularities in liver surface, and moderate amount of ascites, which were suggestive of liver cirrhosis (Fig. 1). The size of spleen was 10cm in long axis. Esophagogastroduodenoscopic examination showed straight and slightly enlarged tortuous varices in distal esophagus (Fig. 2A, B, C), designated as F1 or F2 according to the general rules for recording endoscopic findings of esophagogastric varices [14].
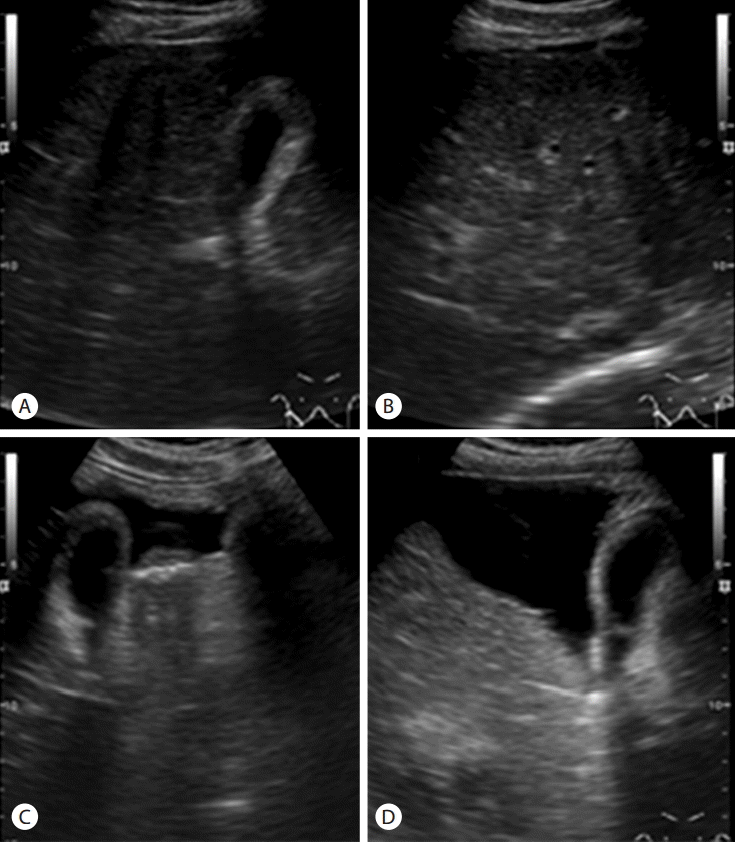
Abdominal ultrasonographic findings. Intercostal and transverse sonograms (A, B) show coarse parenchymal echogenicity, surface nodularity, and a moderate amount of ascites in the perihepatic space. Subcostal oblique sonograms (C, D) show a large amount of ascites in the widened interlobar fissure, which is considered a typical finding of liver cirrhosis.

Esophagogastroduodenoscopic findings. Straight to slightly enlarged (A, B) and tortuous varices (C) were observed on the lower esophagus. The esophageal varices had decreased to minimal varices after 2 years of entecavir therapy (D), and had completely disappeared after 4 years of entecavir therapy (E, F).
Entecavir of 0.5 mg/day orally per day was initiated on April 12, 2010. Spironolactone was also started at this time and continued until ascites and pitting edema resolved. Routine laboratory tests, including liver function tests and HBV DNA level were performed every 1-3 months. HBeAg, anti-HBe, AFP, and abdominal ultrasonography were performed every 6 months. Alanine aminotransferase (ALT) were normalized after 3 months of entecavir therapy. Undetectable HBV DNA (< 20 IU/mL) was achieved after 6 months of entevavir therapy. Serum ALT showed persistent normal value and HBV DNA was undetectable until the last follow up (November 18, 2014).
Follow-up endoscopy, performed on February 9, 2012 and November 28, 2014, showed minimal esophageal varices (Fig. 2D) and complete regression of esophageal varices (Fig. 2E, F), respectively.
DISCUSSION
In patients with liver cirrhosis, esophageal varices are present in approximately 50%, and patients without varices develop them at a rate of 5-8% per year [15-17]. Variceal bleeding, the mortality rate is approximate 15-30% [18-21], occurs at a yearly rate of 5-15% [17,22].
Histological changes in liver cirrhosis have been believed to be irreversible for a long time [23]. However, recent studies conducted by Marcellin et al. demonstrated that 74% of patients with HBV-related liver cirrhosis no longer had cirrhosis after 5 years of tenofovir therapy [8].
Nevertheless, little data are available if complications of cirrhosis, such as esophageal varices can regress after antiviral therapy. Several studies reported spontaneous regression of esopahageal varices in patient with spontaneous HBsAg loss or abstinence from alcohol drinking [24,25] and Koga et al. reported 3 cases whose esophageal varices regressed after lamivudine therapy [26]. Recently, we also reported a case, whose esophageal varices regressed after 3 years of sustained virological response following interferon plus ribavirin combination thrapy [27]. To the best of our knowledge, this is a first report about regression of esophageal varices during entecavir therapy in patients with HBV-related liver cirrhosis.
In conclusion, considering previous results [24-27] and the results of this study, complications of liver cirrhosis, including esophageal varices, can regress after sustained viral suppression in patients with HBV related liver cirrhosis in some patients. Well-designed prospective cohort studies are warranted in near future.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
CHB
chronic hepatitis B, HBeAg, hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HBV
hepatitis B virus
NA
nucleos(t)ide analogues