A case of portal hypertension by presumed as plexiform neurofibroma at the hepatic hilum
Article information
Abstract
Neurofibromas can occur anywhere in the body, but they usually involve the head, neck, pelvis, and extremities. Abdominal visceral involvement is rare, and intrahepatic involvement is even less common. We describe a patient who suffered from plexiform neurofibromatosis with liver involvement. A 49-year-old man, who had previously been diagnosed with neurofibromatosis, underwent esophagogastroduodenoscopy and abdominal ultrasonography for screening purposes. Esophagogastroduodenoscopy showed grade 2 esophageal varices and abdominal ultrasonography showed conglomerated nodules with echogenic appearances in the perihepatic space. Magnetic resonance imaging showed presumed plexiform neurofibroma involving the lesser sac and hepatic hilum and encasing the common hepatic artery celiac trunk and superior mesenteric artery left portal triad. We report an unusual case of portal hypertension attributed to the compressive narrowing of the portal vein by presumed as plexiform neurofibroma at the lesser sac and hepatic hilum.
INTRODUCTION
Neurofibromatosis type 1 (NF1) is a multisystem autosomal dominant disorder that primarily affects the skin and nervous system and is associated with a wide range of signs and symptoms [1]. Neurofibromatosis is classified into two varieties. The most common variety is NF1, also known as Von Recklinghausen disease. This condition is characterized by cutaneous neurofibromas, skin fold freckles, and café-au-lait spots, and may also commonly involve lisch nodules in the iris, optic gliomas, dysplasia of the long bones, and plexiform neurofibromas (PNFs). NF1 may manifest in various ways, depending on the organ involved. Neurofibromatosis type 2, in contrast, involves the development of meningiomas, gliomas, schwannomas, acoustic neuromas, and juvenile posterior subcapsular lenticular opacity [2].
PNFs are benign nerve sheath tumors that develop in individuals with NF1. PNFs can occur anywhere in the body, but usually involve the head, neck, pelvis, and extremities. Abdominal PNFs are most commonly found in the abdomino-pelvic wall and retroperitoneum. Intrahepatic infiltration is rare, comprising 2.3% of all PNFs of the abdomen and pelvis, and has been reported in fewer than 20 cases in the literature, three of which were children [3,4]. In our present case, we describe a patient who suffered from presumed plexiform neurofibromatosis encasing the portal vein and causing portal hypertension with its corresponding complications.
CASE REPORT
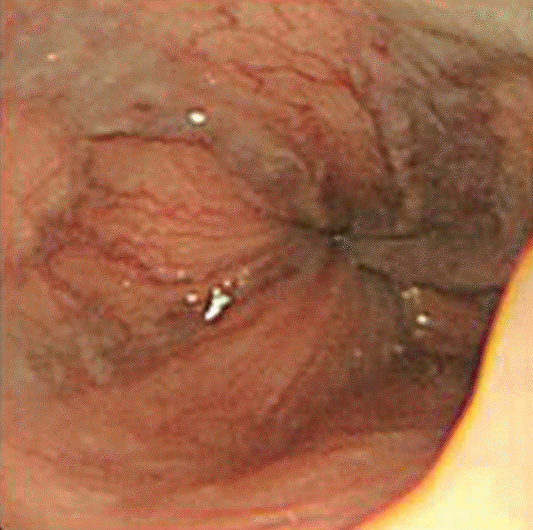
A 49-year-old man without any personal or familial history of relevant diseases except NF1 underwent esophagogastroduodenoscopy and abdominal ultrasonography for screening purposes. The endoscopy showed grade 2 esophageal varices, and abdominal ultrasonography showed normal liver parenchyme, spleno megaly and conglomerated nodules with echogenic appearances in the perihepatic space (Fig. 1, 2). He was therefore referred to our hospital for further evaluation.
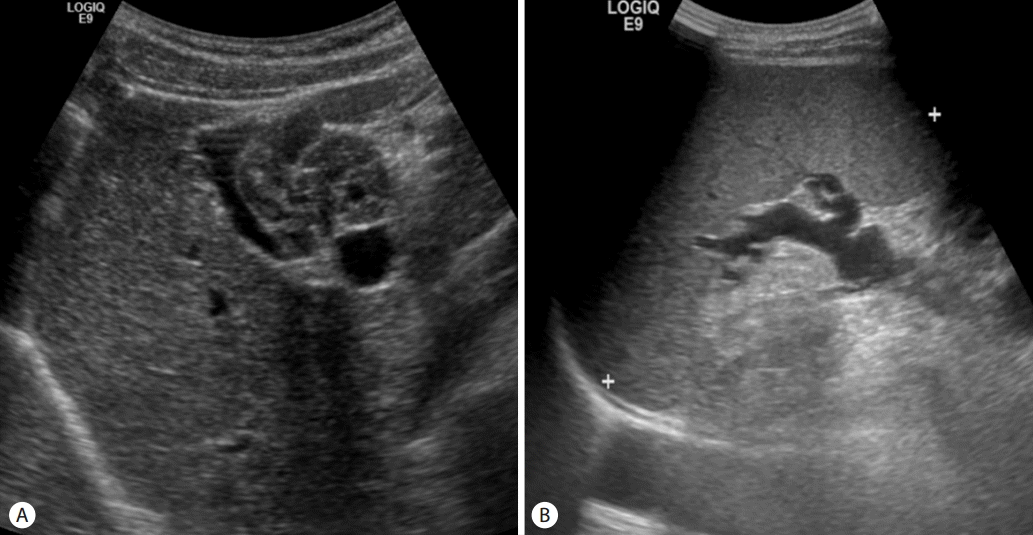
Abdominal ultrasonography. (A) Conglomerated nodules with echogenic appearances in the perihepatic space and normal liver prenchyme. (B) Splenomegaly, suggestive of portal hypertension.
He did not drink alcohol or use tobacco and had not experienced any weight loss or significant abdominal pain. He also denied having melena, hematochezia or any recent changes in his bowel movements. A clinical examination revealed 15 café-au-lait spots >1.5 cm in diameter, multiple cutaneous neurofibromas, and instances of skin fibrosis in the trunk and lower extremities (Fig. 3).

Trunk of patient. Multiple “Cafe-au-lait” spots (arrow) and cutaneous neurofibroma (arrowheads) in chest (A) and back (B).
Both the full blood count and liver function tests were normal: white blood cell, 6.6×103/mm3 (normal range 4.0–10.0×103/mm3); platelet, 219×103/mm3 (normal range 4.0–10.0×103/mm3); aspartate transaminase, 19 IU/L (normal range 0–40 IU/L); alanine transaminase, 16 IU/L (normal range 0–40 IU/L); gamma glutamyltranspeptidase, 27 IU/L (normal range 15–101 IU/L); alkaline phosphatase, 208 IU/L (normal range 136–361 IU/L); and total bilirubin, 1.00 mg/d (Lnormal range 0.10–1.50 mg/dL). A test for the hepatitis B surface antigen showed non-reactive results, and a test for the hepatitis B antibody showed reactive results and a test for the hepatitis C antibody was non-reactive. Autoantibody and hemoglobinopathy screens were negative, and normal levels of alpha-fetoprotein and prothrombin induced by vitamin K absence-II were also found.
Liver computed tomography showed serpentine low-attenuating mass-like lesion with weak enhancement involving lesser sac and hepatic hilum with compressive narrowing of the extrahepatic portal vein in the hepatoduodenal ligament. Liver computed tomography additionally demonstrated a hepatosplenomegaly but without evidence of chronic liver disease (Fig. 4). Liver magnetic resonance imaging showed the presence of a low attenuating mass-like lesion, with weak enhancement found in T2-weighted images and hypointensity found in T1-weighted images. The tumor involved the lesser sac and hepatic hilum, encasing the common hepatic artery, celiac trunk and the superior mesenteric artery left portal triad, suggesting PNF. A central target sign and whorled appearance within the lesion were not observed (Fig. 5). Biopsies are required to confirm PNF, but we could not have histologic confirmation because the patient didn’t want any invasive procedure.
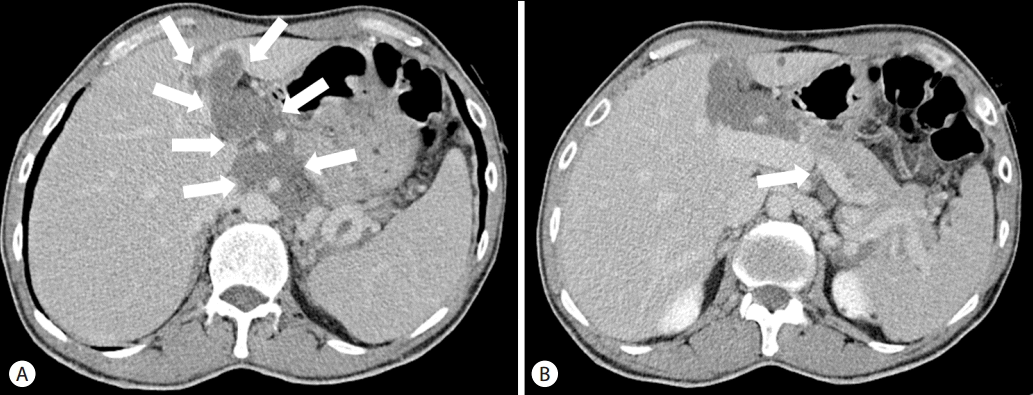
Portal phase of liver dynamic computed tomography showing serpentine low-attenuating mass-like lesion with weak-enhancement involving lesser sac and hepatic hilum (arrows) (A) which compressing the portal vein in the hepatoduodenal ligament (arrow) (B).
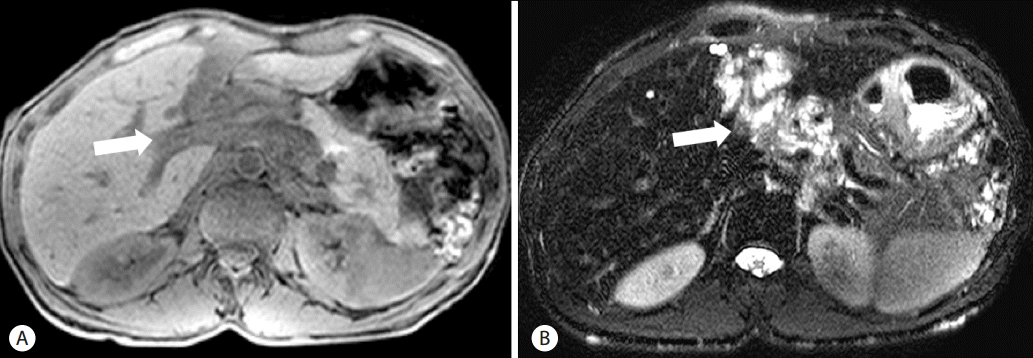
Liver MRI showing mass-like lesion involving the lesser sac and the hepatic hilum (arrows) which is compressing the portal vein. (A) Heterogenous high SI on T2WI. (B) Intermediate low SI on T1WI. MRI, magnetic resonance imaging; SI, signal intensity
Despite the risk of malignant transformation of the tumor and portal hypertension, we started medical therapy with the goal of portal hypertension decompression (beta blocker therapy; propranolol) instead of surgical resection. This choice was made because it was determined that removing the tumor entirely would not be feasible due to its extension into the lesser sac and hepatic hilum.
DISCUSSION
NF1, which has also been referred to as von Recklinghausen disease, is a multisystem autosomal dominant disorder primarily involving the skin and nervous system and occurring with a prevalence of one case per 3,000 live births [5]. NF1 is diagnosed based on clinical criteria. The presence of at least two diagnostic criteria is necessary for a secure diagnosis of NF1. The diagnostic process involves a thorough physical and neurological examination along with a skin examination assessing the presence of skin-fold freckles, café-au-lait macules, and neurofibroma, as well as a slit-lamp to determine whether lisch nodules are present on the iris. Areas of enhanced T2 signal intensity may also be present, along with optic gliomas [6].
Patients with NF1 have a mean life expectancy 10-15 years lower than average, and malignant tumors are the most significant cause of early death in these patients [7]. In patients with NF1, the most pathognomic tumors are neurofibromas, which develop from cells in the nerve sheath and consist of fibroblasts, Schwann cells, mast cells and perineural cells. The primary difference between PNFs and cutaneous fibromas is that an expanded extracellular matrix is found in plexiform fibromas, although the same cell types are present in both conditions [8]. In patients with NF1, PNF has been reported to occur with an incidence of 15-30% [9,10]. PNF involves cells that proliferate along the nerve sheath, forming multiple fascicles and branches that may stretch across the entire length of a nerve [5]. There is an increased risk of PNFs developing into malignant peripheral nerve sheath tumors (MPNSTs). It is clear that most, if not all, MPNSTs arise from preexisting PNFs, metastasize throughout the body, and often have a poor prognosis [6]. Ducatman et al. showed that the five-year survival rate in patients with NF1 and MPNSTs was 16% [11]. The prognosis is showed no relationship with radiation or chemotherapy treatments, but did correlate with the tumor size and extent of resection. Wanebo et al. conducted a study reviewing 28 patients diagnosed with MPNSTs, of whom 15 had NF1 [12]. Their findings also indicated that survival did not correlate with radiation or chemotherapy, but was influenced by the performance of surgical resections.
PNFs appear in computed tomography imaging as multi-lobulated masses with low attenuation that generally occur within a single area of nerve distribution. In magnetic resonance imaging, the tumor shows a low signal compared to the liver on T1-weighted imaging, with signal intensity similar to that of muscle. On T2-weighted sequences, the tumor shows high signal intensity with areas of heterogeneity. A central target sign and a whorled appearance within the lesion may also be noted [13], but this did not occur in our case. However, imaging alone is often insufficient to assess the malignant transformation of PNFs into MPNSTs [14]. Biopsies are therefore required to securely diagnose MPNSTs, although sampling error remains a potential difficulty. Despite the malignant transformation, we could not have histologic confirmation, because the patient didn’t want any invasive procedure.
Abdominal PNFs are rare and most commonly found in the abdomino-pelvic wall and retro-peritoneum. Intrahepatic infiltrations comprise 2.3% of all PNFs in the abdomen and pelvis; fewer than 20 cases have been reported in the literature, of which three have occurred in children[3,4]. In our present case, we describe a patient who suffered from plexiform neurofibromatosis encasing the portal vein and causing portal hypertension with its corresponding complications.
It is possible for internal PNFs to persist stably for many years without resulting in any clinical problems. In such cases, PNFs are usually detected incidentally during imaging studies carried out for some other purpose. In patients with neurologic deficits or clinical symptoms, it can be problematic to remove PNFs because the tumor is likely to have infiltrated the surrounding tissues and nerves.
The optimal timing for surgical intervention in such cases has been the focus of considerable controversy. It has been argued that early surgery is necessary to prevent major impairments, but it has also been argued that the complete removal of even small PNFs is rarely feasible, such that recurrence after surgery remains a real possibility [14]. In the present case, the patient was treated with medication instead of surgical resection despite the risk of malignant transformation of the tumor and portal hypertension. This decision was made because it was determined that removing the tumor entirely would not be feasible due to its extension into the lesser sac and hepatic hilum.
In conclusion, we report an unusual case of portal hypertension attributed to the compressive narrowing of the portal vein by presumed as plexiform neurofibroma at the lesser sac and hepatic hilum. It is important to note that plexiform neurofibroma involving the hepatic hilum can develop portal hypertension, and patients with plexiform neurofibroma at the hepatic hilum require tests for the portal hypertension.
Acknowledgements
The authors thank Dr. Jae Gu Jung for his insightful and valuable comments during the preparation of this manuscript.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
MPNST
malignant peripheral nerve sheath tumor
NF1
neurofibromatosis type 1
PNF
plexiform neurofibroma