Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma
Article information
Abstract
Background/Aims:
Several studies have suggested that surgical resection (SR) can provide a survival benefit over transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) at the intermediate stage according to the Barcelona Clinic Liver Cancer (BCLC) staging system. However, the criteria for SR remain to be determined. This study compared the long-term outcome of intermediate-stage HCC patients treated by either TACE or SR as a primary treatment modality, with the aim of identifying the patient subgroup that gained a survival benefit by either modality.
Methods:
In total, 277 BCLC intermediate-stage HCC patients treated by either TACE (N=225) or SR (N=52) were analyzed.
Results:
The overall median survival time was significantly better for SR than TACE (61 vs. 30 months, P=0.002). Decision-tree analysis divided patients into seven nodes based on tumor size and number, serum alpha-fetoprotein (AFP) level, and Child-Pugh score, and these were then simplified into four subgroups (B1–B4) based on similarities in the overall hazard rate. SR provided a significant survival benefit in subgroup B2, characterized by ‘oligo’ (2–4) nodules of intermediate size (5–10 cm) when the AFP levels was <400 ng/ml, or ‘oligo’ (2–4) nodules of small to intermediate size (<10 cm) plus a Child-Pugh score of 5 when the AFP level was ≥400 ng/mL (median survival 73 vs. 28 months for SR vs. TACE respectively; P=0.014). The survival rate did not differ significantly between SR and TACE in the other subgroups (B1 and B3).
Conclusion:
SR provided a survival benefit over TACE in intermediate-stage HCC, especially for patients meeting certain criteria. Re-establishing the criteria for optimal treatment modalities in this stage of HCC is needed to improve survival rates.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world, and the third leading cause of cancer–related death worldwide [1]. As any other malignancies, evidence-based, proper staging and decision for treatment determines the outcome of HCC patients. The Barcelona-Clinic Liver Cancer (BCLC) staging system is the most widely used and accepted staging system for HCC that is endorsed by the European Association for the Study of the Liver (EASL) and the American Association for the Study of the Liver Disease (AASLD) [2,3]. BCLC staging system divides HCC patients into 5 stages (0, A, B, C and D), and allocates treatment strategy accordingly [2,3].
In BCLC staging system, intermediate stage (B) is defined as asymptomatic, multinodular tumors without an invasive pattern (multinodular tumor, Child-Pugh A-B and performance status 0), and transarterial chemoembolization (TACE) is recommended as a first-line treatment [2,3]. TACE has been reported to extend the survival of these patients [4], nonetheless, outcome prediction has been heterogeneous for BCLC B, and sub-staging of BCLC stage B is being suggested to further refine BCLC stage B [5]. Furthermore, surgical resection (SR) was applied to certain groups of patients with intermediate HCC, with an improved survival by SR over TACE [6-8]. However, TACE was proven to be as effective as SR among operable HCC [9], indicating that recommending resection for all operable HCC is not a good approach for intermediate stage HCC.
While it is likely that a certain group of BCLC intermediate stage HCC may gain survival benefit from SR over TACE, optimal selection criteria for SR in BCLC intermediate stage is not clear. More data are needed to determine the best candidates for each treatment modality. Therefore, this study was conducted to compare long-term outcome of intermediate stage HCC patients treated by either TACE or SR as a primary treatment modality, and identify subgroup which gains survival benefit by either modality.
MATERIAL AND METHODS
Study population
For this study, we used HCC registry of Samsung Medical Center, Seoul, Korea. This prospective registry enrolled treatment naïve, newly-diagnosed HCC patients who received care at Samsung Medical Center, Seoul, Korea since January 2005. When patients were newly diagnosed with HCC, well-trained abstractors collected the patient’s data including age at diagnosis, gender, date of diagnosis, etiology, liver function (e.g., Child-Pugh class), tumor characteristics (e.g., number of tumors, maximal tumor size, presence and extent of portal vein invasion, and type of extrahepatic spread), tumor stage, and initial treatment modality, in a prospective manner. HCC was diagnosed either histologically or clinically according to the regional guideline [10].
We screened a total of 3,514 patients who were registered at HCC registry between January 1, 2005 and December 31, 2009. We included 303 patients who were diagnosed at BCLC intermediate stage. Among them, we excluded 26 patients who met the following exclusion criteria: 1) patients who were not treated by either SR or TACE (25 patients) and 2) those who had double primary cancer (1 patient). The final study population was comprised of 277 BCLC intermediate stage HCC patients who were treated by either SR (SR group, n=52) or TACE (TACE group, n=225). The study was conducted in accordance with the Declaration of Helsinki, and was reviewed and approved by the Institutional Review Board at Samsung Medical Center (IRB No: 2015-06-136). Because the study was based on the retrospective analysis of existing administrative and clinical data, the Institutional Review Board waived the requirement of informed patient consent.
Collection of data and primary end-point assessment
All the information was prospectively collected in computer databases before this analysis. We used demographic, clinical, laboratory data that were collected from each patient at the time of HCC diagnosis: age, gender, etiology of liver disease, serum alphafetoprotein (AFP) levels and Child–Pugh class with score. From the radiologic findings, tumor characteristics were evaluated regarding size, number, extent, and presence of vessel invasion. The primary end-point was overall survival, which was defined as the time from the primary treatment for HCC to death. All patients were followed-up from the baseline till March 2015. Patients’ survival data were collected from electronic medical records and national death statistics (Statistics Korea, KOSTAT), and all death at the time of survival assessment were certified.
Treatment and follow-up
TACE was performed in the following manner. After selective arteriography of the superior mesenteric, celiac, and common hepatic arteries using a 5-French catheter, the hepatic artery was catheterized with a coaxial microcatheter. After the microcatheter was positioned into or as close as possible to the tumor feeding branch, an emulsion of doxorubicin hydrochloride (adriamycin, Il-dong, Seoul, Korea) and iodized oil (Lipiodol; Guerbet, Aulnaysous-Bois, France) was slowly infused through the catheter. Oily TACE was performed as selectively as possible and a microcatheter was routinely used. The doses of iodized oil and doxorubicin were determined based on the size and vascularity of the tumor; the maximum doses of iodized oil and doxorubicin for a single session of TACE being 25 mL mg and 70 mg, respectively. Infusion of the lipiodol mixture was followed by particulate embolization with 1- to 2-mm-diameter gelatin sponge pledgets (Cutanplast, MasciaBrunelli, Milan, Italy). SR was performed by surgeons experienced in hepatobiliary surgery. The hepatectomy was performed in a standardized manner. After TACE or surgical resection, patients were followed up every 1-3 months during the first 2 years and at 3-6 months interval thereafter. After treatment, patients were evaluated for treatment response, and liver function. For patients who experienced recurrence, subsequent treatment was applied if clinically feasible, by respective physicians.
Statistical analyses
Continuous variables were summarized as mean±standard deviation or median and ranges, and compared using Student’s t-test or the Mann-Whitney U-test, respectively. Categorical variables were summarized as the number of events for each category and percentage, and compared using the χ2 or Fisher exact test, as appropriate. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox-regression analysis was conducted for factors associated with survival. Multiple Cox regression analysis was carried out using variables that showed association in univariate analysis with a p-value <0.2. Decision tree analysis was conducted to determine sub-groups with similar survival expectancy. All statistical analysis was performed with the Statistical Analysis System version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
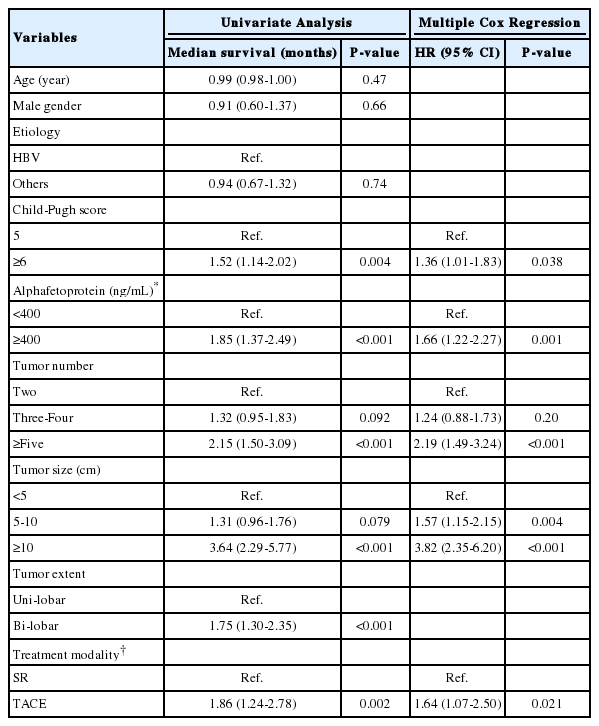
The comparison of characteristics between the TACE and SR groups were shown in Table 1. Patients in the SR group were younger and had better liver function (more patients with Child-Pugh class A, lower prothrombin time and higher albumin level). Tumor number was <4 in majority of patients (98.1%) in the SR group, while 76.0% of the TACE group had <4 of tumor number (P<0.001). Uni-lobar involvement was more common in the SR than in the TACE group (78.8% vs. 35.6%, P<0.001). There were no significant differences in gender, maximal tumor size, serum AFP levels, and etiology of liver disease.
Survival of TACE and SR group
The median survival was 32 months, and 1-, 3-, and 5-year survival rate of 80.8%, 44.0% and 32.3%, respectively. During a median 30 months of follow up (range: 0-116 months), 28 (53.8%) patients in the SR group and 167 (74.2%) patients in the TACE group died. Overall survival was significantly better in the SR group than TACE group (median survival 61 vs. 30 months, P=0.002). The 1-, 3-, and 5-years survival rate were significantly better in the SR group than TACE group (92.3%, 65.0% and 51.8% vs. 78.2%, 39.2% and 27.9%, respectively; P<0.001) (Fig. 1). Child-Pugh score, AFP levels, tumor number, tumor size and initial treatment modality were factors that were independently and significantly associated with patient survival after a multiple Cox regression (Table 2).
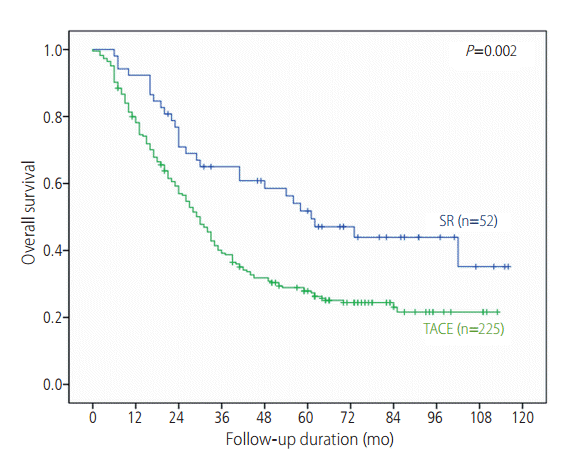
Survival curves for the SR and TACE groups. Patient survival was significantly better for SR. SR, surgical resection; TACE, transarterial chemoembolization.
Identifying survival nodes with similar survival expectancy
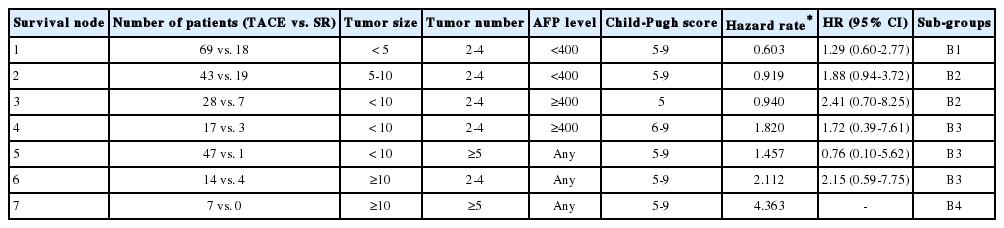
In decision tree analysis, patients were first divided by tumor size followed by tumor number. Patients were further divided based on AFP levels and Child-Pugh score (Fig. 2). The characteristics and estimated hazard rates of each node were summarized in Table 3. The survival of each node was shown in Figure 3. Based on estimated hazard rates, patients were re-classified into 4 subgroups (Table 3).
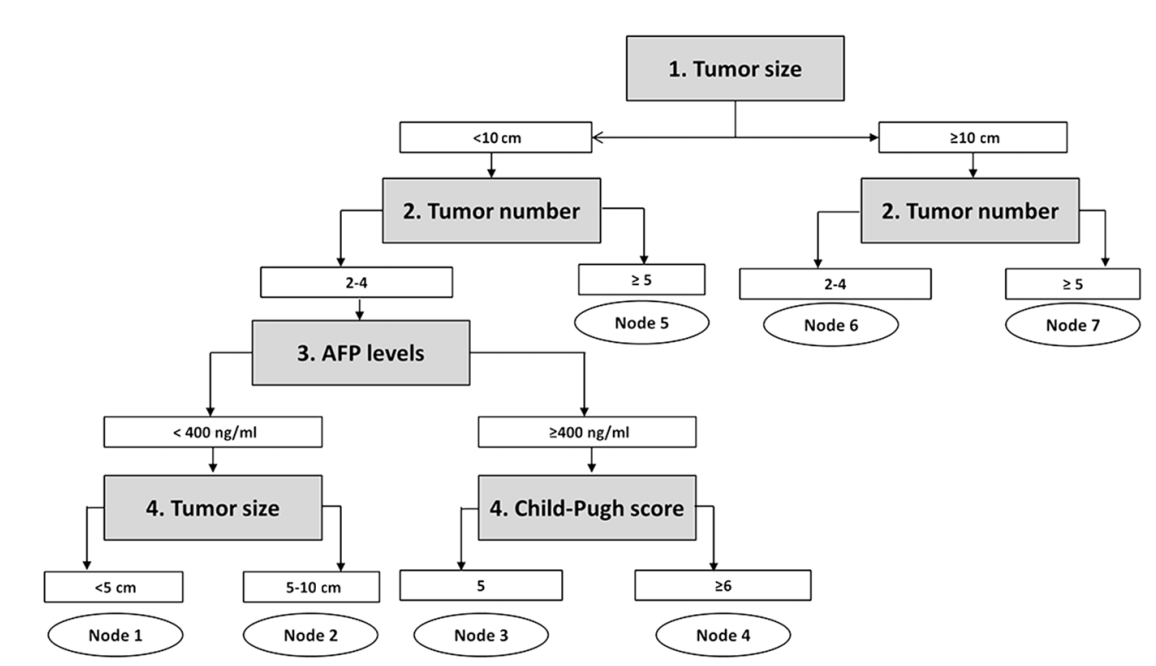
Subgroups obtained by decision-tree analysis. Decision-tree analysis divided patients into seven nodes based on tumor size and number, serum AFP level, and Child-Pugh score. AFP, alphafetoprotein.
Survival in each subgroup according to the treatment modality
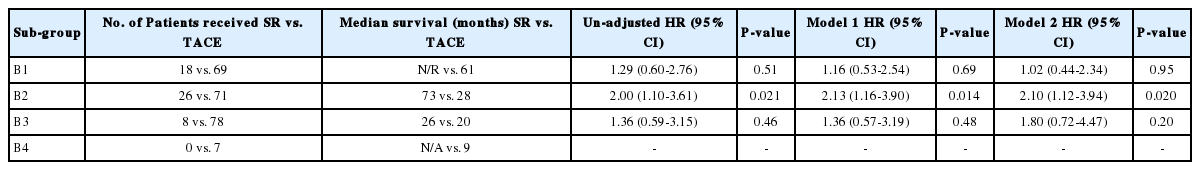
The overall survival was significantly different by subgroups (median survival: 62, 30, 21 and 9 months for B1, B2, B3 and B4, respectively, P<0.001). The survival was not significantly different between SR and TACE in subgroup B1 (median survival: all survived beyond 50th percentile vs. 61 months; 5-year survival rate: 57.8% vs. 50.1% for SR vs. TACE, respectively, P=0.51) and in sub-group B3 (median survival: 26 vs. 20 months; 5-year survival rate: 15.0% vs. 12.7% for SR vs. TACE, respectively, P=0.46). The survival was significantly better in the SR than TACE in subgroup B2 (median survival: 73 vs. 28 months; 5-year survival rate: 53.6% vs. 26.1%; for SR vs. TACE, P=0.021). In subgroup B4, none of the patients received SR and all patients received TACE with median survival of 9 months (Table 4).
DISCUSSION
TACE is the recommended first-line treatment for intermediate stage HCC in BCLC staging system [2,3]. Current guidelines recommend SR only for single nodules of any size in patients without tumor-related symptoms and clinically significant portal hypertension and with normal bilirubin [2,3]. However, in this study, the overall survival was significantly better in the SR group than TACE group (median 61 vs. 30 months), indicating that SR can be a better treatment modality than TACE in intermediate stage HCC. Consistent with this study, the BRIDGE study, a multiregional cohort study, reported that non-ideal resection candidates based on current guidelines who received embolization showed higher hazard ratio for survival (1.257, 95% confidence interval = 1.109-1.429) than those who received resection [11]. In the recently proposed Hong Kong Liver Cancer (HKLC) classification, Yau et al., also reported superior survival by resection over TACE (5-year survival probability, 52.1% vs. 18.7%) in BCLC-B stage patients classified as HKLC-II patients [12]. In a randomized controlled trial involving patients for resectable multiple HCCs beyond Milan criteria, SR showed better 3-year overall survival rate (51.5%) than TACE (18.1%, P<0.001) [13]. Thus, it is likely that a certain subgroup of patients in intermediate stage might gain survival benefit by SR over TACE [8]. With advances in surgical techniques, improved peri- and post-operative management, new drugs for hepatitis C virus, and nucleos(t)ide analogs for hepatitis B virus, the controversy of resecting non-ideal candidate by current guidelines is increasing [14].
Although SR may provide survival benefit in patients with intermediate stage HCC [15], it is not applicable to all patients when technically feasible. Among operable HCC, TACE was proven to be as effective as SR in selected patients [9]. Tumor removal in patients with a large tumor burden and/or impaired liver function may not be worth attempting, as survival could even be decreased [14]. Hence, there is a need for establishing criteria that can identify patients who will gain survival benefit from SR over TACE in intermediate stage HCC. In this study, we used a decision-tree analysis to identify patients with similar survival expectancy (Fig. 2). The results of this analysis indicated that patients in intermediate stage have a heterogeneous population with different survival expectancy.
The decision-tree analysis showed that tumor size and number are the main factors that determined patient survival. Patients with huge (≥10 cm, node 6 and node 7) or multiple nodules (≥5 nodules, node 4 and node 7) showed shorter survival than those with small to intermediate (<10 cm) sized and ‘oligo’ (2-4 nodules) tumors. Among patients with small to intermediate (<10 cm) sized, ‘oligo’ (2-4 nodules) tumors, serum AFP levels and Child-Pugh score further classified patients survival. Based on survival expectancy, patients could be re-classified into 4 subgroups (B1, B2, B3 and B4). Subgroup B1 was characterized as small sized (<5 cm), ‘oligo’ tumors (2-4 nodules) with low AFP levels (<400 ng/ml). They showed the best survival (median: 62 months) among intermediate stage HCC. Subgroup B4 was characterized by huge size (≥10 cm) and multiple (≥5) tumors, and showed worst survival (median: 9 months). Subgroup B2 consisted of patients with 2 characteristics: 1) intermediate sized (5-10 cm) and ‘oligo’ tumors (2-4 nodules) with low serum AFP (<400 ng/ml), and 2) small to intermediate (<10 cm) sized, ‘oligo’ tumors (2-4 nodules) with high serum AFP (≥400 ng/ml) and well-preserved liver function (Child-Pugh score 5). Subgroup B3 consisted of patients with 3 characteristics: 1) huge size (≥10 cm) but ‘oligo’ (2-4) tumors, 2) small to intermediate (<10 cm) sized but multiple (≥5) tumors, 3) small to intermediate (<10 cm) sized, ‘oligo’ (2-4) tumors with high AFP (≥400 ng/ml) and Child-Pugh score ≥6. Median survival was 30 and 21 months for subgroup B2 and B3, respectively.
In this study, SR showed significant survival benefit for subgroup B2 (73 vs. 28 months, P=0.021), but not in subgroups B1 and B3. No patient received SR in subgroup B4 (Table 4). Several important features among characteristics of patients in subgroup B2 should be considered in choosing candidates for SR in intermediate stage HCC. First, tumors were small to intermediate sized (<10 cm) and ‘oligo’ (2-4 nodules) numbered in stage B2. It has been suggested that ‘oligometastases’ is a distinct clinical entity that refers to restricted tumor metastatic capacity, with the implication that local cancer treatments can be curative in a proportion of patients [16,17]. The importance of ‘oligo’ nodules in selecting patients for SR has been demonstrated by others as well. Jianyong et al., compared SR or TACE in intermediate stage HCC and reported that SR showed better 5-year survival rate than TACE (65.1% vs. 50.3%, P=0.002) for BCLC stage B HCC patients with 2 to 3 tumors, but no significant difference in 5-year survival rate (40.8% vs. 21.6% for SR vs. TACE, P=0.064) for BCLC stage B HCC patients with more than 3 tumors [18]. Second, among potentially ‘operable’ HCC (nodes 1,2,3 and 4: small to intermediate sized, ‘oligo’ nodules), serum AFP levels was the next determinant in the decision-tree model. The level of AFP is a well-known predictor for survival and high AFP is associated with aggressive tumor behavior [19]. Low AFP levels, small tumor, low tumor number and good liver function were factors that predicted good survival after TACE [20]. This may explain why TACE yielded similar survival benefit to SR in subgroup B1 (node 1: ‘oligo’ nodule, small sized (<5 cm), low AFP levels). When any of poor prognostic factors for TACE was present (e.g., elevated AFP levels or large size), SR showed survival benefit over TACE. These data indicate that SR should be considered as the first-line option for subgroup B2 of intermediate stage HCC, characterized by ‘oligo’ (2-4) nodules, intermediate sized (5-10 cm) when AFP levels is <400 ng/ml or small to intermediate sized (<10 cm) plus Child-Pugh score 5 when AFP level is ≥400 ng/ml. For subgroups B1 and B3, similar survival rate was observed by TACE or SR. In these cases, treatment decision between TACE and SR should be selected after considering the patient’s preference, cost, complications related to either treatment, even when HCC are judged to be ‘operable’.
There were several limitations in this study. Sample size of SR cases was small (n=52). Decision to operate non-ideal candidates for resection by current guidelines is influenced by factors such as the extent of a planned resection and perceived risk of the intervention [14], which we could not adequately control in this study. It should be noted that some of the patients who are classified as subgroup B2 cannot undergo SR due to location of tumor or other factors. Most patients in this cohort were mainly composed of hepatitis B virus (HBV)-related HCC patients. For HBV-related HCC patients, potent and safe nucleos(t)ide analogs that can preserve liver function are available [21], and a large proportion of HBV-related HCC patients are not accompanied by cirrhosis [22].
Despite these limitations, this study confirmed that intermediate stage HCC is composed of heterogeneous population with different survival expectancy, which argues for the needs of sub-classification of this stage. In intermediate stage HCC, SR yields survival advantage over TACE, especially for those meeting certain criteria (subgroup B2 in this study). Re-establishing criteria for optimal treatment modalities in intermediate stage HCC is needed to improve survival.
Acknowledgements
This study was supported by Samsung Medical Center grant CRP1500083.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
HCC
hepatocellular carcinoma
TACE
transarterial chemoembolization
BCLC
the barcelona clinic liver cancer
AFP
alphafetoprotein
EASL
European association for the study of the liver
AASLD
American association for the study of the liver disease
SR
surgical resection
HKLC
Hong Kong liver cancer